Discovery of a new liquid-liquid interfacial deformation by partial miscibility
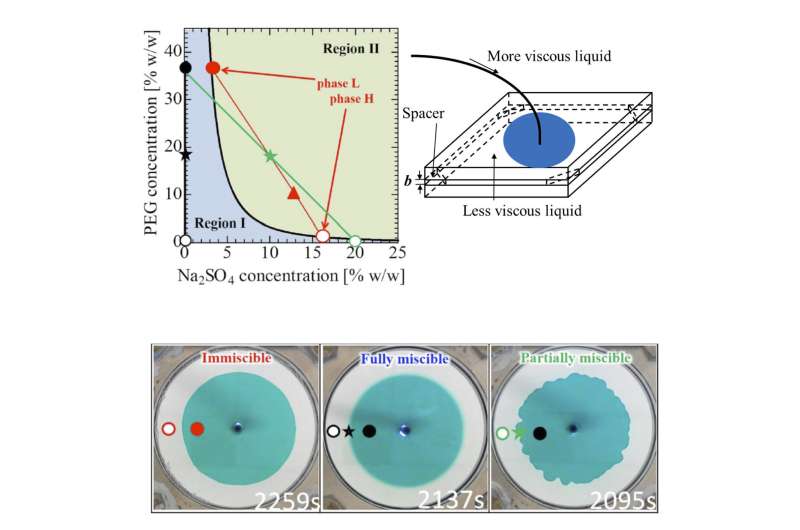
The international collaborative team of Tokyo University of Agriculture and Technology (TUAT, Japan), IIT Ropar (India), Osaka Univ. (Japan) has discovered that "partially miscibility," in which two liquids do not mix completely with finite solubility, is capable of deforming the liquid-liquid interface. This interfacial deformation originates due to the spontaneous motion driven by phase separation between the soluble species, and is a phenomenon that cannot be seen with completely mixed (fully miscible) with infinite solubility or (almost) immiscible with no solubility.
The researchers published their results in Physical Review Fluids on Oct 29th, 2019.
The process of displacing one fluid in a porous medium by injecting another fluid is important in reactions and separations in chemical processes, as well as in enhanced-oil-recovery and CO2 sequestration. In particular, when a less viscous fluid displaces a more viscous fluid, the interface between the two fluids becomes hydrodynamically unstable and deforms in a finger shape. This phenomenon is called "viscous fingering," and it has been studied since the 1950s as one fluid dynamics problem. Now, it is widely known that the properties of these two fluids can be traditionally classified according to whether they are fully miscible or immiscible. On the other hand, when a more viscous fluid displaces a less viscous fluid, classically the interface stably spreads, regardless of whether the two fluids are fully miscible or immiscible.
"It has long been known that two fluids are partially miscible in underground processes with high-pressure conditions, such as oil recovery and CO2 storage, "said Dr. Nagatsu, corresponding author on the paper and an associate professor in the Department of Chemical Engineering at TUAT. "However, the intricate understating of interfacial dynamics in partially miscible systems has not been well-studied. One of the reasons is that fluid displacement research has been mainly conducted by fluid mechanics researchers so far, and they missed finding the experimental systems that are partially miscible at room temperature and atmospheric pressure."
The research team succeeded in changing the miscibility of the system to fully miscible, immiscible, and partially miscible with little change in the viscosities of high-viscosity or low-viscosity liquids at room temperature and atmospheric pressure by using an aqueous two-phase system consisting of polyethylene-glycol (PEG), sodium-sulfate, and water and by changing the salt (sodium-sulfate) concentration (see Fig. a).
"We found that a new interfacial deformation is observed in the case where the two liquids are partially miscible (see Fig. c) when a more-viscous liquid displaces a less-viscous one in a Hele-Shaw cell (Fig. b) which is a model that mimics flow in porous media. This is very much counter-intuitive because no deformation takes place in such a situation when the two fluids are fully miscible or immiscible (Fig. b). We showed this interfacial instability originates due to spontaneous flow driven by phase separation between the soluble species," Nagatsu explains.
"Our result shows that the effect of partial miscibility of liquids on interfacial hydrodynamics is not in the middle of fully miscible and immiscible, but has completely different properties. We emphasize that this will open a new cross-disciplinary research area involving hydrodynamics and chemical thermodynamics. Also, the displacement with partial miscibility in a porous medium takes places in the oil recovery process from the formation and the CO2 injection process into the formation, and thus our finding is expected to contribute to improve the accuracy of the phenomena prediction of those processes," adds Nagatsu.
More information: Ryuta X. Suzuki et al, Fingering pattern induced by spinodal decomposition in hydrodynamically stable displacement in a partially miscible system, Physical Review Fluids (2019). DOI: 10.1103/PhysRevFluids.4.104005
Provided by Tokyo University of Agriculture and Technology





















