Credit: RUDN University
RUDN chemist synthesized iron (II) 3-D coordination polymer, the first coordination compound of iron, assembled from substituted nicotinic acid H2cpna. This compound can be used in production of catalysts, necessary for oxidative functionalization of saturates. This is the process essential for petroleum refining. The article was published in Crystals.
The processes for developing materials for gas storage and separation of complex mixtures are often based on coordination polymers. Structural features also allow researchers to use them as conductors, wherein inorganic and conjugated organic spacers can carry electric current. In commercial scale, coordination polymers are used as colorants. Depending on the type of metal atom in the coordination polymer, different tones of colors can be obtained. In addition, coordination polymers can be used as efficient catalysts for different chemical transformations, which include the functionalization of hydrocarbons for the synthesis of added-value products.
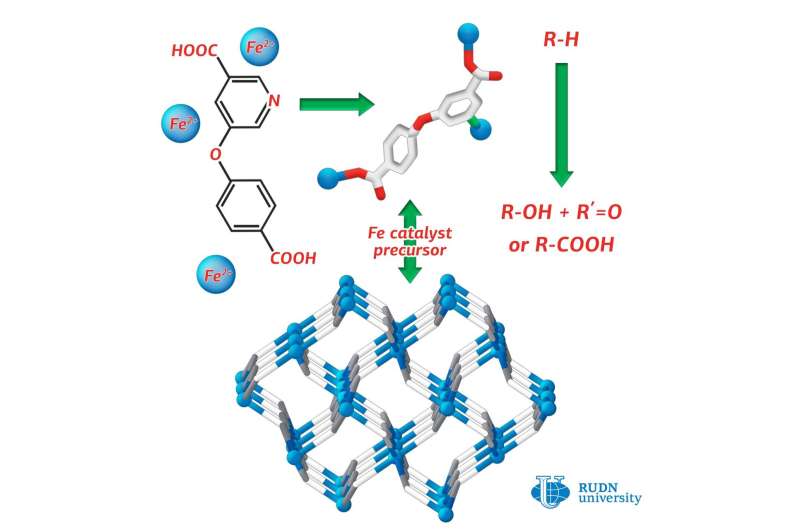
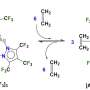
Coordination polymers are compounds consisting of metal atoms and organic ligands. Often they are more stable than pure organic substances. RUDN chemist Alexander Kirillov used a derivative of nicotinic acid (H2cpna) as the building block in synthesis, while iron atoms fulfilled the role of metallic center. Substituted nicotinic acid may act as the ligand. It contains one phenyl and one pyridine ring, which are bound together by an ether functional group.
For synthesis of Fe(II) coordination polymer, the reaction was carried out under hydrothermal conditions between ferrous sulphate (II) in water and H2cpna at the temperature 160 ºС. The synthesis lasted for three days. To prove the structure and the characteristics of the resulted product X-ray diffraction and other methods were applied.
The catalytic effect of the substance for different reactions was explored. Alexander Kirillov from RUDN conducted oxidation and carboxylation of propane and cyclic alkanes under mild conditions. The reaction yield was equal to 23 percent. For comparison, in the industrial oxidation of cyclohexane to cyclohexanol and cyclohexanone (products used in the production of plastics) the product yield has been only 5 to 10 percent.
Investigation of the catalytic activity of the coordination polymer obtained by RUDN chemists proved that it can be used for catalysis of processes such as the oxidative fictionalization saturated hydrocarbons, leading to greater product yields in reactions under mild conditions.
More information: Na Zhao et al. Synthesis, Structural Features, and Catalytic Activity of an Iron(II) 3D Coordination Polymer Driven by an Ether-Bridged Pyridine-Dicarboxylate, Crystals (2019). DOI: 10.3390/cryst9070369
Provided by RUDN University