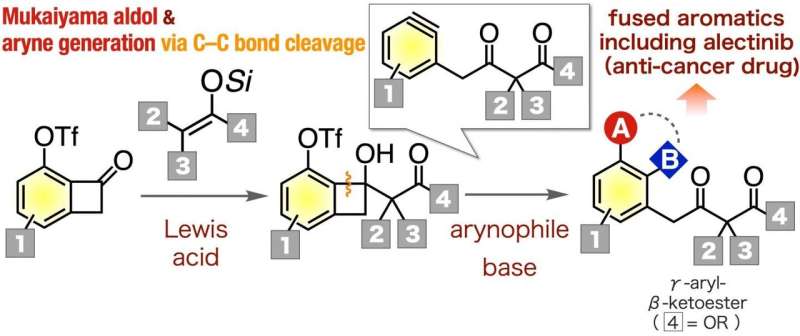
The Mukaiyama aldol reaction of 6-(triflyloxy)benzocyclobutenones with ketene silyl acetals and subsequent generation of γ-aryl-β-ketoester-type arynes from resulting 6-(triflyloxy)benzocyclobutenols in the presence of arynophiles efficiently provided a wide range of γ-aryl-β-ketoesters. The method was applicable to the synthesis of an analog of ALK inhibitor. Credit: Department of Chemical Bioscience,TMDU
Researchers at the Tokyo Medical and Dental University (TMDU) have introduced a new synthetic process for producing an important family of carbon-based molecules known as γ-aryl-β-ketoesters. These molecules are used in the production of many vital pharmaceuticals, including alectinib, which is administered to treat non-small-cell lung cancer, and Januvia, a diabetes drug. This chemical approach may help in the preparation of a diverse range of their analogs and many other medication candidates more quickly.
Organic chemistry, which studies reactions involving carbon-based molecules, is central to the pharmaceutical industry. Certain reactions, such as the formation of multi-substituted aromatic compounds, are essential to the production of a variety of drugs. One important class of molecules that can be utilized as versatile intermediates are the γ-aryl-β-ketoesters. However, it has been up till now difficult to synthesize a variety of these critical molecules.
In a study published in Organic Letters at October 24, researchers from Tokyo Medical and Dental University (TMDU) report a new reaction pathway to easily produce γ-aryl-β-ketoesters. To do this, they utilized aryne chemistry, which involves the removal of two substituents from a benzene ring, yielding a very reactive chemical species. "To successfully synthesize the γ-aryl-β-ketoesters, we decided to use a pathway that involves γ-aryl-β-ketoester-type arynes, because they are useful intermediates for creating multi-substituted aromatic derivatives," says first author Keisuke Uchida.
As a demonstration of the value of producing γ-aryl-β-ketoester using this novel method, the research team synthesized an analog of alectinib, which is an important inhibitor of certain lung cancers. As a complex molecule, the synthesis of various analogs by the conventional method takes considerable time and efforts, so the new approach that renders various γ-aryl-β-ketoesters easily available can improve the accessibility to them. This is true for many other organic compounds as well.
"By virtue of the flexibility of aryne intermediates, our new synthetic approach may assist in the preparation of many important bioactive compounds, both for the pharmaceutical sector as well as for agrochemical sciences," senior author Takamitsu Hosoya says. The research group plans to expand the scope of their method to other molecules which may lead to faster and more cost-effective drug discovery in the future.
More information: Keisuke Uchida et al, Synthesis of Diverse γ-Aryl-β-ketoesters via Aryne Intermediates Generated by C–C Bond Cleavage, Organic Letters (2019). DOI: 10.1021/acs.orglett.9b03418
Journal information: Organic Letters
Provided by Tokyo Medical and Dental University