Using a molecular motor to switch the preference of anion-binding catalysts
Many organic molecules are chiral, which means that they are non-superimposable on their mirror image. Those mirror images are called enantiomers and can have different properties when interacting with other chiral entities, for example, biomolecules. Selectively producing the right enantiomer is therefore important in, for example, pharmaceuticals. University of Groningen chemists Ruth Dorel and Ben Feringa have now devised a method that not only achieves this but that also controls which version is being produced using light. The results were published online by the journal Angewandte Chemie on November 17.
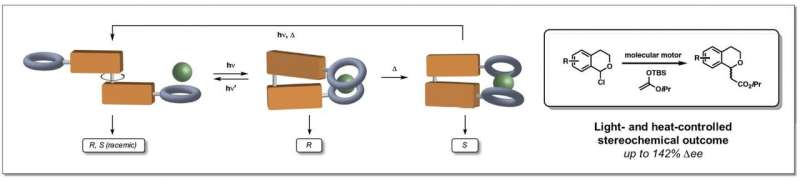
The process is based on the use of a molecular motor created by Professor Feringa, for which he was awarded the 2016 Nobel Prize in Chemistry. The motor molecule was used to produce the first switchable catalyst for asymmetric anion-binding catalysis. Dr. Ruth Dorel explains: "We attached anion-binding arms on both sides of the motor molecule to create an anion receptor that can act as a catalyst. This receptor will adopt a helical structure in the presence of anions that, depending on the relative position of the arms, will exist in different forms."
Switch
In this study, a very slow-turning motor molecule was used so that different stages of the rotation cycle could be used in catalysis. The molecular motor is made up of two identical halves, linked by a double carbon-carbon bond that acts as the axle. By sequentially exposing the molecule to UV-light and heat, unidirectional rotation around the axle is achieved. Consequently, the anion-binding groups on both halves of the motor are able to switch from being apart from each other (trans) to being in close proximity on the same side of the motor molecule (cis). In the cis configuration, the arms can adopt two different configurations leading to two different helices with opposite handedness. Dorel: "The helicity dictates the enantiomer of the product that this catalyst will produce."
Drugs or polymers
The new catalyst was tested on a benchmark reaction for anion-binding catalysis. "We now have a proof of principle," Dorel explains. Practical applications are a long way off but could potentially be found both in fundamental research and in the production of drugs or polymers. For many drugs, only one of the two mirror images is the active substance—the other may do nothing, or even cause side effects. "And in polymer production, a catalyst like this could alter the shape and properties of the polymer chain on demand."
More information: Ruth Dorel et al, Stereodivergent Anion Binding Catalysis with Molecular Motors, Angewandte Chemie International Edition (2019). DOI: 10.1002/anie.201913054
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by University of Groningen