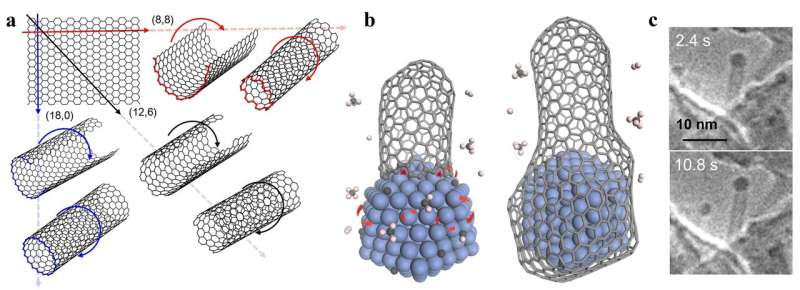
(a) Carbon nanotubes (CNTs) could be viewed as single-atom layer thick graphene sheets rolled into a cylinder. Different directions of rolling determine CNTs' properties. (b) Schematic diagram showing a carbon nanotube's lifetime during chemical vapor deposition synthesis. Transition metals (blue structure) serve as catalysts, critical to elongate the CNT (left), until the carbon concentration on the catalyst surface becomes so abundant that the nanoparticle gets encapsulated by graphitic or amorphous carbon, forming a "cap" at the end of the cylinder and ending the growth of the CNT (right). (c) Environmental transmission electron microscope images of a CNT taken at different times during growth. The CNT contains a cobalt nanoparticle on its top end, a typical feature of tip-growth. Credit: IBS
In a recently published paper in Science Advances, Feng Ding of the Center for Multidimensional Carbon Materials and colleagues have achieved the creation of a specific type of carbon nanotubes (CNTs) with a selectivity of 90 percent, and expanded the current theory that explains the synthesis of these promising nanocylinders.
CNTs are incredibly strong and light nanomaterials made of carbon with superior current carrying capacity and very high thermal conductivity, making them ideal for electronic applications. Although CNTs are considered as some of the most interesting materials for the future, scientists are still struggling for their controllable synthesis.
The shape of CNTs can be compared to paper tubes—just as a cylinder can be created by rolling a sheet of paper, so CNTs can be imagined as a single layer of graphite rolled up on itself. Differently shaped tubes can be produced by rolling a paper around its long side, its short side, or diagonally at angles. Depending on the rolling direction, a graphite layer can produce different CNT structures, some are conducting and others are semiconducting; thus, selectively creating a specific type of CNT will be key for future applications, such as energy efficient computer chips. However, CNTs are not produced by rolling, but are grown nanometer by nanometer, adding carbon at the rim of nanocylinders, one atom at a time. However, to date, the understanding on CNT growth remains very limited and rational experimental design for the growth of specific types of CNTs is challenging.
One of the most promising manufacturing methods for CNTs is chemical vapor deposition (CVD). In this process, metal nanoparticles combined with carbon-containing gases form CNTs inside a high-temperature furnace. On the tip of the tubes, the metal nanoparticles play a critical role as catalysts: They dissociate the carbon source from the gases, and assist the attachment of these carbon atoms to the CNT wall, making the tubes longer. The growth of the CNT terminates once the catalyst particle is encapsulated by graphitic or amorphous carbon.
Carbon atoms are inserted onto the interface between a growing CNT and a catalyst nanoparticle in active sites of the rim, and are available to incorporate new atoms. A previous model of the CNT growth rate showed that the latter is proportional to the density of these active sites at the interface between CNT and the catalyst, or the specific structure of the CNT.
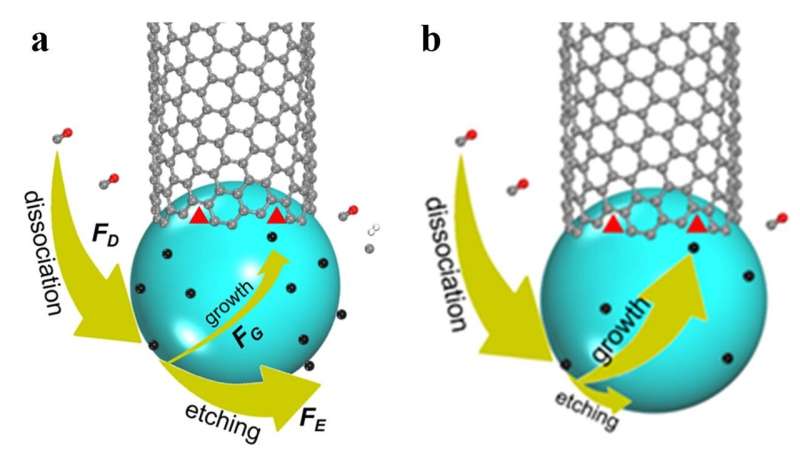
The model for carbon nanotube (CNT) growth in the (a) presence and (b) absence of sufficient etching agents. In (a) most dissociated carbon atoms are taken away from the catalyst surface by etching agents and the CNT growth will depend on the number of active sites (red triangles) or the structure of the CNT. In (b), in the absence of etching agent, every decomposed carbon atom has to be a part of the CNT, and therefore, the number of active sites or the structure of the CNT has no impact on the growth rate, but will affect the duration of CNT's grow. Credit: IBS
In this study, the researchers monitored the steady growth of CNTs on a magnesium oxide (MgO) support with carbon monoxide (CO) as the carbon feedstock and cobalt nanoparticles as catalysts at 700 degrees C. The direct experimental measurements of 16 CNTs showed how to expand the previous theory. "It was surprising that the growth rate of a carbon nanotubes only depends on the size of the catalyst particle. This implies that our previous understanding of carbon nanotube growth was not complete," says Maoshuai He, the first author of the paper.
More specifically, carbon atoms that are deposited on the catalyst particle surface can be either incorporated on the active side of the CNT or removed by etching agents, such as H2, H2O, O2, or CO2. To explain the new experimental observations, the team included the effects of carbon insertion and removal during CNT growth and discovered that the growth rate depends on the catalyst's surface area and tube diameter ratio.
"Compared to the previous model, we added three more factors: the rate of precursor deposition, the rate of carbon removal by etching agents, and the rate of carbon insertion into a carbon nanotube wall. When feedstock dissociation cannot be balanced by carbon etching, the rate of carbon nanotube growth will no longer depend on the structure of the carbon nanotube. On the other hand, the previous theory is still valid if the etching is dominating," explains Ding, a group leader of the Center for Multidimensional Carbon Materials.
Interestingly, the new theory of CNT growth leads to a new mechanism to selectively grow a specific type of CNTs, denoted as (2n, n) CNTs, which is characterized by the maximum number of active sites at the interface between the CNT and the catalyst. This CNT structure would correspond to rolling a sheet of graphite diagonally at an angle of around 19 degrees.
"If there is no carbon etching and the carbon nanotube growth is slow, carbon atoms on the catalyst surface will accumulate," says Jin Zhang, co-author of the study and professor of Peking University, China. "This may lead to the formation of graphitic or amorphous carbon, which are established mechanisms of carbon nanotube growth termination. In this case, only carbon nanotubes which are able to add carbon atoms on their walls—that is, with the highest number of active sites—can survive."
Guided by the new theoretical understanding, the researchers were able to design experiments that produced (2n, n) CNTs with a selectivity of up to 90 percent: the highest selective growth of this type of CNT was achieved in the absence of any etching agent and with a high feedstock concentration.
More information: Maoshuai He et al. Growth kinetics of single-walled carbon nanotubes with a (2n, n) chirality selection, Science Advances (2019). DOI: 10.1126/sciadv.aav9668
Journal information: Science Advances
Provided by Institute for Basic Science