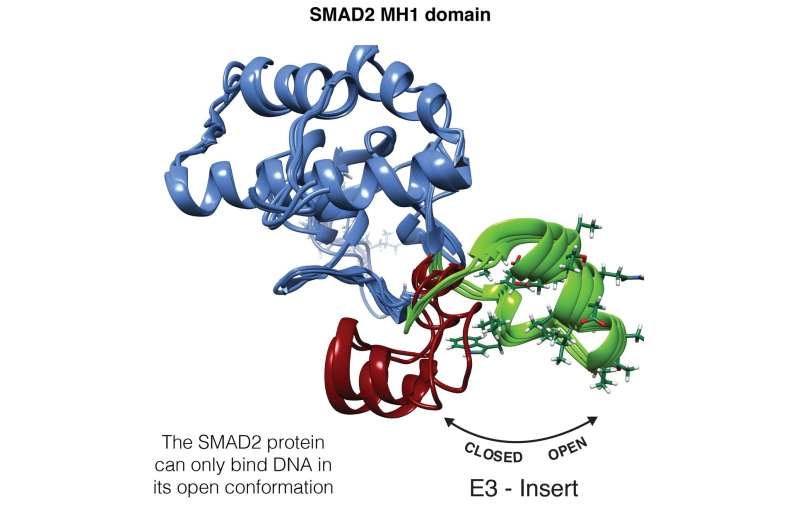
The SMAD2 protein can have two orientations. Green indicates the open configuration that allows DNA binding and red the closed configuration, which is incompatible with such binding. Credit: Maria J. Macias, IRB Barcelona
Scientists at the Institute for Research in Biomedicine (IRB Barcelona), in collaboration with the Sloan Kettering Institute (New York, U.S.), have published the structural and functional keys that distinguish two very similar transcription factors, namely SMAD2 and SMAD3.
SMADs form a family of proteins that regulate the expression of genes related to processes such as embryo development, cell regeneration and immune response. In spite of being almost identical, SMAD2 is essential for embryo development while SMAD3 is expendable.
"In this study, we have been able to answer why two proteins, SMAD2 and SMAD3, that have such similar sequences perform such different roles during embryo development," explains María J. Macias, ICREA researcher and head of the Structural Characterization of Macromolecular Assemblies Laboratory at IRB Barcelona.
Tri-dimensional change in the SMAD2 protein, from an open configuration that allows the transcription factor to bind to DNA (in green) to a closed configuration that is incompatible with such binding (in red). Credit: Pau Martín-Malpartida and Maria J. Macias, IRB Barcelona
Using a multidisciplinary approach involving a combination of molecular biology, cellular biology, developmental biology and structural biology expertise, this team of researchers has discovered that the key to the difference between the two transcription factors lies in a 30 amino acid region of SMAD2, which is absent in SMAD3.
"Because their sequences are so similar, it was difficult to study SMAD2 and SMAD3 separately with the tools available until now and their functions were confused. The fine-tuning of high-resolution techniques such as nuclear magnetic resonance and crystallography has allowed us to differentiate between the two," says Macias, who emphasizes that "this study is the result of 5 years' of work by the scientists in the two teams."
Refuting belief: SMAD2 does bind to DNA
The adoption of an open structure by a 30 amino acid segment present in SMAD2—which is absent in SMAD3—allows direct DNA binding and activates the expression of key genes in development. This finding thus refutes the theory that has been accepted to date that SMAD2 is a transcription factor that does not bind to DNA.
"We have observed that when SMAD2 is correctly folded it is able to interact directly with the double helix of DNA. The study of the structure of SMAD2 has revealed that it can undergo a tridimensional change, which leads to two orientations of the protein, one compatible with DNA binding and the other not," comments Eric Aragón, a member of Macias' team and co-first author of the article.
More information: Eric Aragón et al. Structural basis for distinct roles of SMAD2 and SMAD3 in FOXH1 pioneer-directed TGF-β signaling, Genes & Development (2019). DOI: 10.1101/gad.330837.119
Journal information: Genes & Development
Provided by Barcelona Institute of Science and Technology