Researchers obtain the first mice born with hyper-long telomeres
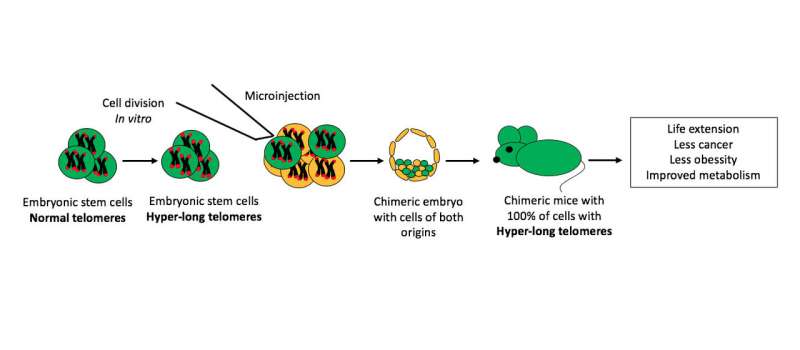
A chance finding 10 years ago led to the creation by researchers of the Spanish National Cancer Research Centre (CNIO) of the first mice born with much longer telomeres than normal in their species. Telomeres shorten throughout life, so older organisms have shorter telomeres. Given this relationship between telomeres and aging, the scientists launched a study generating mice in which 100 percent of their cells had hyper-long telomeres. The findings are published in Nature Communications and show only positive consequences: The animals with hyper-long telomeres live longer and in better health, free from cancer and obesity. This marks the first time that longevity has been significantly increased without any genetic modification.
"This finding supports the idea that, when it comes to determining longevity, genes are not the only thing to consider," says Maria Blasco, head of the CNIO Telomeres and Telomerase Group and intellectual author of the paper. "There is margin for extending life without altering the genes."
Telomeres form the ends of chromosomes in the nucleus of each cell in the body. Their function is to protect the integrity of the genetic information in DNA. Whenever the cells divide the telomeres, they are slightly shortened, so one of the main characteristics of aging is the accumulation of shorter telomeres in cells. "Telomere shortening is considered to be one of the primary causes of aging, given that short telomeres cause aging of the organism and reduce longevity," the authors write in a paper published in Nature Communications.
The CNIO Telomeres and Telomerase Group has already shown in diverse studies that preventing the shortening of telomeres through the activation of the telomere-lengthening enzyme telomerase extends longevity without any secondary effects.
However, until now, all interventions on the length of telomeres have been based on altering the expression of genes through one technique or another. In fact, some years ago, the CNIO group developed a gene therapy that fosters the synthesis of telomerase, obtaining mice that live 24 percent longer without developing cancer of other illnesses associated with age.
13 percent longer, slimmer and free from cancer
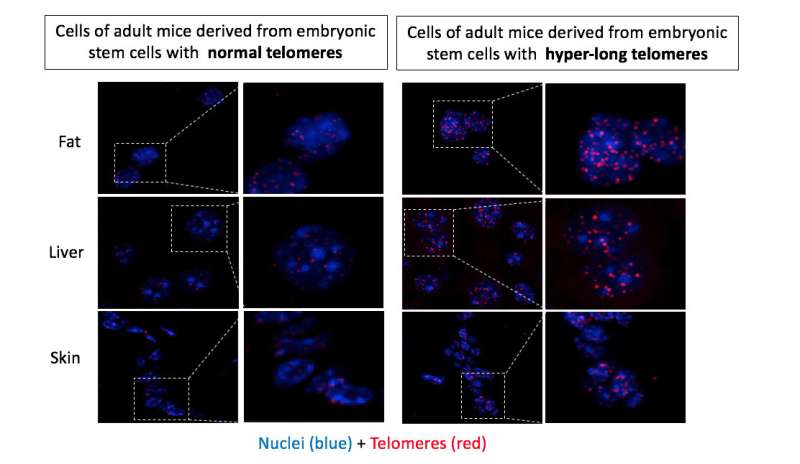
The most significant result is that there has been no genetic alteration in the mice born with hyper-long telomeres. In 2009, researchers worked with so-called IPS cells—cells from an adult organism that have pluripotency, or the capacity to generate a full organism—and they observed that after a certain number of divisions in culture plates, these cells acquired telomeres twice as long as normal. Intrigued, they confirmed that the same occurred in normal pluripotent embryonic cells as they are kept in cultivation after being removed from the blastocyst.
On researching the phenomenon, Blasco's team found that during the pluripotency stage, there are certain biochemical marks (epigenetic marks) on the telomeric chromatin that facilitate their lengthening by the telomerase enzyme. For this reason, the telomeres of pluripotency cells in cultivation were extended to twice the normal length.
The question was whether the embryonic cells with hyper-long telomeres could produce live mice. Some years ago, the group demonstrated that they could in research also published in Nature Communications. However, these first animals were chimerical—in other words, only between 30 percent and 70 percent of their cells came from embryonic cells with hyper-long telomeres. Their good health may be attributed to the proper functioning of the rest of the cells with normal telomeres.
In the study that has now been published, the authors induced hyper-long telomeres in 100 percent of mice cells, so this entire peculiar feature is attributable to this phenomenon. Indeed, there are many peculiarities.
"Unprecedented results"
"These mice have less cancer and live longer," the authors write. "An important fact is that they are slimmer than normal because they accumulate less fat. They also show lower metabolic aging, with lower levels of cholesterol and LDL (bad cholesterol), and an increased tolerance to insulin and glucose. Damage to their DNA as they age is less, and their mitochondria, another Achilles heel of aging, function better."
In conclusion, "these unprecedented results show that longer than normal telomeres in a given species are not harmful but quite the contrary: They have beneficial effects, such as increased longevity, delayed metabolic age and less cancer."
More specifically, the average longevity of mice with hyper-long telomeres is 13 percent higher than usual. The metabolic alterations observed are also relevant, as this is the first time that a clear relationship between the length of telomeres and metabolism has been found. The genetic route of insulin and glucose metabolism is identified as one of the most important in relation to aging.
However, what is most striking for researchers is that this finding paves the way for extending longevity without changing the genes of the organism. Biochemical changes in the telomeric chromatin that facilitates the lengthening of telomeres in the pluripotency phase is epigenetic, or in other words, it acts as a chemical annotation that modifies the work of genes, but does not alter their essence.
"Extending the time during which embryonic cells remain in pluripotency to generate mice with longer telomeres, protected from cancer and obesity, and with increased longevity, has been enough to make mice have longer telomeres and live longer," the authors write. "We present a new model of mice in which aging has been delayed without any genetic manipulation."
More information: Mice with hyper-long telomeres show less metabolic aging and longer lifespans. Miguel A. Muñoz-Lorente, Alba C. Cano-Martin, Maria A. Blasco, Nature Communications, 2019. DOI: 10.1038/s41467-019-12664-x
Journal information: Nature Communications
Provided by The Spanish National Cancer Research Centre