Columbia University researchers have captured new detailed images of a temperature-sensing molecule in its open, intermediate, and closed states. The findings will help us understand the mechanics of hot, warm, cool, and cold sensation and accelerate the development of drugs for variety of conditions, including inflammatory skin disease, itch, and pain.
The findings were published online in Nature Structural and Molecular Biology.
Researchers have known for more than a century that temperature and pain perception are controlled by specialized sensory neurons. In the late 1990s, scientists discovered a superfamily of molecules, called transient receptor potential (TRP) channels, inside these neurons. Of the 28 known TRP channels, 11 are highly sensitive to changes in temperature. These "thermoTRPs" open and close in response to temperature fluctuations, allowing ions to pass through and transmit signals to the central nervous system.
"ThermoTRPs act as biological thermometers, allowing organisms to sense temperatures in the entire physiological range, from noxious cold to noxious heat," says study leader Alexander I. Sobolevsky, Ph.D., associate professor of biochemistry and molecular biophysics at Columbia University Vagelos College of Physicians and Surgeons. "How these channels sense temperature and then subsequently undergo changes at the molecular level has remained a puzzle."
An important breakthrough in the field came in 2013 when a technique called cryo-electron microscopy (cryo-EM) was first used to image representatives of the TRP channel family. Since then, almost every type of TRP channel has been imaged with this technique. But scientists were unable to capture cryo-EM images of thermoTRP channels at different temperatures—particularly high temperatures, where heat-sensitive channels are activated.
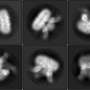
Using cryo-EM, scientists at Columbia University have revealed structural changes in the temperature-sensing molecule TRPV3 when activated by heat. Credit: Sobolevsky Lab, Columbia University Vagelos College of Physicians and Surgeons
In the new study, Sobolevsky's lab identified a mutation in one of these channels, TRPV3, that increases its sensitivity to temperature. By exploiting this mutation, his team was able to fix the channel in open, sensitized, and closed positions—an essential step in understanding a protein's structure using cryo-EM imaging. The resultant images revealed the structural changes that happen in TRPV3 once it is activated by heat.
"These structural changes seem to originate from the ion-conducting membrane portion of TRPV3 that senses temperature through its interaction with the surrounding membrane lipids," says Appu K. Singh, Ph.D., an associate research scientist in biochemistry and molecular biophysics at Columbia University and first author of the paper. "Further studies are needed to identify the temperature sensor of these channels more precisely."
TRPV3 is mainly expressed in skin cells. The channel plays a role in a variety of physiological functions, including temperature sensation, nociception (the nervous system's response to certain harmful or potentially harmful stimuli), itch, maintenance of the skin barrier, wound healing, and hair growth. TRPV3 is also associated with numerous human skin diseases, such as atopic dermatitis, psoriasis, and rosacea. Studies show that mice lacking TRPV3 are unable to sense warmer temperatures.
"Our structures not only can serve as a springboard for studies of the biophysical principles of ion channel temperature activation, but also as templates for the design of drugs for a variety of conditions that affect the skin," says Sobolevsky.
In a separate study published in Nature Communications, Sobolevsky's team used cryo-EM to determine the structure of crTRP1, a thermoTRP found in Chlamydomonas reinhardtii, a type of algae. This is the first time the structure of a thermoTRP has been described in a micro-organism.
The paper is titled, "Structural basis of temperature sensation by the TRP channel TRPV3."
More information: Structural basis of temperature sensation by the TRP channel TRPV3, Nature Structural and Molecular Biology (2019). DOI: 10.1038/s41594-019-0318-7 , nature.com/articles/s41594-019-0318-7
Journal information: Nature Communications , Nature Structural and Molecular Biology
Provided by Columbia University Irving Medical Center