Histone modifications are the influencers of zygotic genome awakening
The zebrafish is an important model organism in biology. Humans and zebrafish share 70 percent of their genes, and more than 80 percent of human genes associated with disease are known to have a zebrafish counterpart. Additionally, their entire genome sequence has been identified, and it is more efficient to grow fish than mice.
Their development, however, is a little different from that of mammals. Although fish reproduce sexually, like all vertebrates, fertilization of the egg is external, as both the egg and sperm are released into the water. Despite this important difference, the majority of sequences involved in protein synthesis associated with embryonic development and regulation is conserved across species.
This means that some of the basic building blocks that give cells the instructions of how to build a living organism are conserved across species. Instead of getting rid of these sequences, evolution regulates their expression through modifications of genomic DNA and histone proteins, around which DNA is bound. These "epigenetic factors," as scientists call them, determine whether genes become functional or not because they alter the chemical and/or structural composition of DNA. This regulation of what gets to become what is particularly important when an organism develops.
A group of scientists from Tokyo Tech, led by Professor Hiroshi Kimura, set out to study epigenetic changes during zebrafish development. Professor Kimura says, "The structural conservation of histones and crucial enzymes for transcription (i.e., RNA polymerases II), as well as the fact that zebrafish embryos are transparent, makes them a perfect model to study the role of these modifications in the development of living organisms."
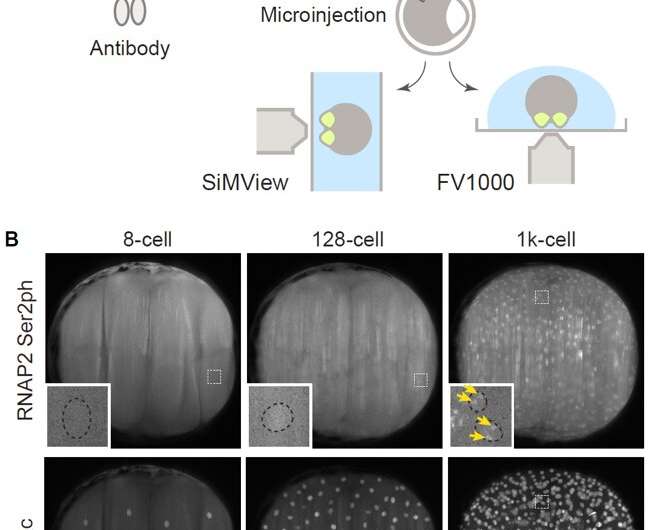
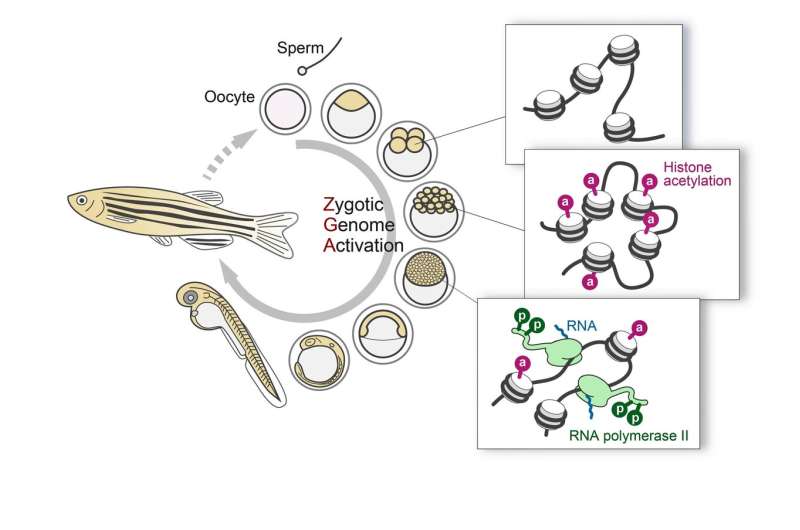
Using fab-based live endogenous modification labeling, or FabLEM, a technique to visualize the dynamics of post-translational modifications using specific antibodies in live cells (Figure 2), the team observed active RNA polymerase II during zygotic genome activation (ZGA), or the process during which the development of the embryo comes under control of the zygote or the developing embryo itself instead of the maternally deposited material. Although the timing of this transition is species-specific, it also occurs in mammals.
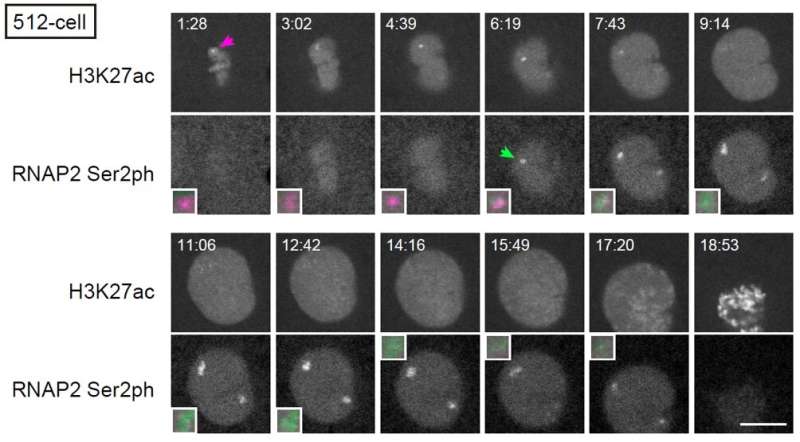
Then the team compared the changes in histone modification with the timing of genome activation. One of the main findings of their studies was that acetylation that occurs on the 27th lysine in histone H3, or H3K27, becomes concentrated prior to active transcription (Figure 3). Visualizing transcripts of the earliest activated gene miR-430 together with H3K27 acetylation, confirmed the findings, and transcription inhibitor did not prevent the accumulation of H3K27 acetylation. What the results suggest is that H3K27 acetylation is an epigenetic factor that awakes the genome for transcription.
Because RNA polymerase II is present in all species, the method can also be applied to study the development in other living organisms. "This opens the possibility of further research on the link between transcription regulation and nuclear organization," remarks Professor Kimura.
More information: Yuko Sato et al, Histone H3K27 acetylation precedes active transcription during zebrafish zygotic genome activation as revealed by live-cell analysis, Development (2019). DOI: 10.1242/dev.179127
Journal information: Development
Provided by Tokyo Institute of Technology