An artist’s depiction of a T cell. Credit: NIAID
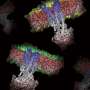
A team of researchers from the Harbin Institute of Technology and Peking University has used single-particle cryogenic electron microscopy to study the human T cell receptor complex. In their paper published in the journal Nature, the group describes their study and what they learned from it.
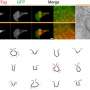
T cells are involved in carrying out an immune response during an infection. Prior research has shown that each T cell has a T cell receptor, which are sensitive to proteins produced by other cells. If certain proteins are detected, the T cell becomes activated and attempts to destroy the abnormal cell. Prior research has also shown that T cell receptors consist of eight proteins, six of which are known as CD3 proteins. The other two are known as TCR proteins. In a given T cell, the TCR proteins exist outside the cell membrane, which means they are the part of the receptor responsible for detecting foreign proteins. The CD3 proteins exist in the cell membrane (in the shape of helices) and serve as a communications system between the cell and the TCR proteins. In this new effort, the researchers used a special type of electron microscopy to learn more about the ways TCR and CD3 proteins interact and communicate.
Prior research has shown that there are two types of TCR proteins, TCRα and TCRβ—and four kinds of CD3 proteins, CD3ε, CD3γ, CD3δ and CD3ζ. Researchers have believed that each type of protein serves a particular role in detecting foreign proteins. The researchers hoped to learn more about the role of each by using a single-particle cryogenic electron microscope with a resolution of 3.7 Angstroms.
The researchers report that their imaging efforts offered credence to theories that suggest T cell receptors behave in a direction-selective way, and do so as mechanosensors when recognizing antigens—they were also found to bind ligands in just one direction. The researchers also learned more about the transmembrane bundle of CD3 helices—they discovered they exist in a parallel orientation relative to the membrane material that serves to replace lipids for solubilizing proteins.
The researchers note that their work could provide a platform for future studies surrounding the immune response as well as autoimmune diseases.
More information: De Dong et al. Structural basis of assembly of the human TCR–CD3 complex, Nature (2019). DOI: 10.1038/s41586-019-1537-0
Journal information: Nature
© 2019 Science X Network