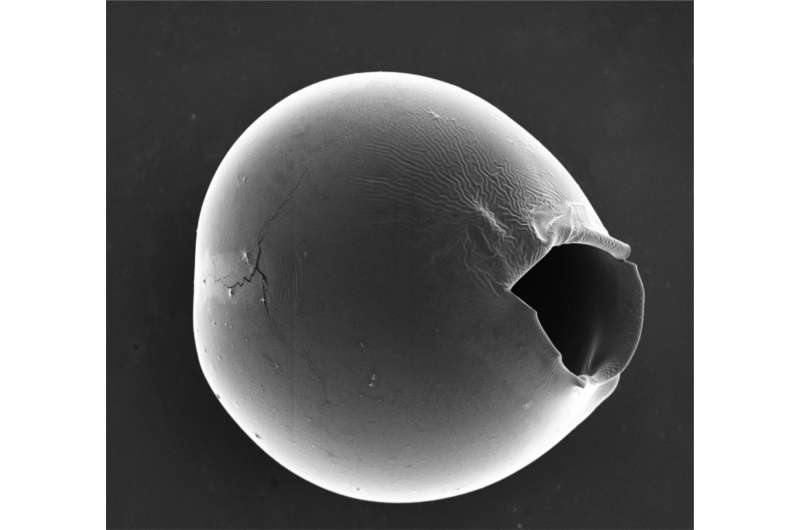
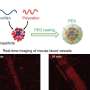
Microcapsules with shells of varying thickness (red) can stably encapsulate cargo and release it at precisely calibrated, low osmotic pressure, allowing safe delivery of drugs and other substances inside the human body. Credit: Wyss Institute at Harvard University
Cracking an egg to release its yolk requires applying external force (like being smacked against the edge of a bowl) to overcome the strength of the eggshell. Similarly, delivering microcapsule-contained therapeutic biomolecules into the human body requires that their containers be broken after they are injected, so that the cargo can be delivered in the right place at the right time. A number of external stimuli can be used to trigger the release of encapsulated molecules, one of the easiest of which is osmotic pressure, as it simply requires the introduction of water to cause the microcapsules to swell and burst. However, in order to create enough inner pressure to break the capsule shell, large amounts of an osmotic agent must be added to the microcapsule to attract the water, and the resulting high burst pressure could damage tissues or cause blood clots.
A solution to this stumbling block has now been developed by researchers at Harvard's Wyss Institute for Biologically Inspired Engineering and John A. Paulson School of Engineering and Applied Sciences (SEAS), who devised a way to create microcapsules with shells of uneven thickness that allows them to burst at lower osmotic pressures, making them safer for use in the human body. The research is published in Small.
"Our shells' weakest part is 40 times thinner than their strongest part, which makes it much easier for them to break and release their cargo," said first author Weixia Zhang, Ph.D., a Postdoctoral Fellow at the Wyss Institute and SEAS. "On the flip side, these microcapsules are exceedingly durable and do not leak if they are not exposed to elevated osmotic pressure, making them very stable and capable of storing their contents for a long time."
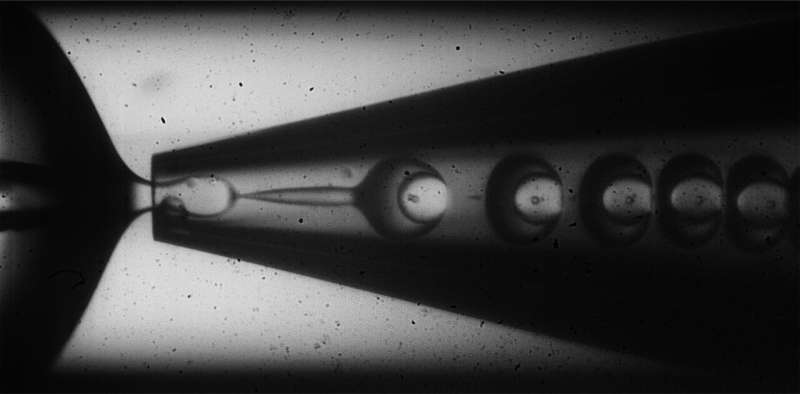
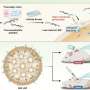
To fabricate their inhomogeneous microcapsules, the researchers used a glass capillary microfluidic device that employed a water-in-oil-in-water method to encapsulate a water solution containing sucrose, an osmotic agent, within a shell of monomers suspended in oil. When the monomers are exposed to UV light, they react with each other and crosslink to form a solid, polymer shell around the sucrose solution. By varying the rates at which the sucrose solution "cargo" and the monomer oil "shell" flow through the device, the team discovered that they could introduce variations in the thickness of the shells that formed, creating lopsided capsules with thicker walls on one side and thinner on the other.
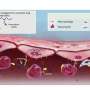
The microcapsules burst at the thinnest part of the shell, leaving an opening tens of microns in size that allows most biomolecules and drugs to be released. Credit: Wyss Institute at Harvard University
The researchers then subjected their microcapsules to osmotic shock by adding water, which diffused into the microcapsules and made them begin to swell at the thinnest part of the shell. After about 20-30 minutes, the thinned shell cracked, forming an opening that was tens of micrometers in size, which is large enough for most biomolecules and nanomaterials to be released successfully. Increasing the difference between the thinnest and the thickest part of the shell led to a greater number of burst microcapsules, confirming that the degree of inhomogeneity impacted the efficacy of cargo release.
"Being able to create microcapsules with high degree of inhomogeneity by altering the shell thickness during the manufacturing process and to release the cargo with much smaller osmotic pressure opens up a new application in controlled release, which is very important for drug delivery in medicine, as well as other fields," said co-first author Liangliang Qu, Ph.D., a Postdoctoral Fellow at the Wyss Institute and SEAS.
To test the microcapsules' durability, the team encapsulated a fluorescent polymer within them and measured the change in fluorescent intensity in their cores over time. They observed no change in intensity for 30 days after encapsulation, demonstrating that the microcapsules retained their cargo without leaking. Furthermore, the polymer's size is much smaller than most biomolecules, such as antibodies and enzymes, suggesting that the shells could be used to protect and store biomolecules or drugs for extended periods of time.
Finally, the researchers co-encapsulated a protease (an enzyme that breaks down proteins) and sucrose inside their microcapsules for 37 days, then applied osmotic shock to trigger the release of their contents. The protease retained 91% of its original activity, demonstrating that this storage method did not significantly impair its biological function.
The microcapsules are created using an oil-in-water-in-oil technique that achieves inhomogeneous shell thickness by varying the flow rates of the shell and cargo materials. Credit: Wyss Institute at Harvard University
"Compared to other controlled release carriers, such as cells, nanoparticles, or vesicles, this system is highly versatile, stable, and customizable, making it an attractive alternative for safely and effectively delivering drug and other biomolecules for human health and other applications," said corresponding author David Weitz, Ph.D., a Core Faculty Member at the Wyss Institute who is also the Mallinckrodt Professor of Physics and Applied Physics at SEAS.
The team is continuing to develop their microcapsules by optimizing the shell material to further decrease the osmotic pressure required to rupture them. They plan to first apply their technology to the delivery of drugs, such as therapeutic antibodies, with the goal of being able to use the human body's high water content to act as the rupture trigger after injection.
"This project is a great example of how simpler solutions can often be better than complicated ones, as the only input needed to burst the microcapsules is mechanical pressure, rather than complex chemistries or molecular switches," said co-author Donald Ingber, M.D., Ph.D., who is the Wyss Institute's Founding Director, the Judah Folkman Professor of Vascular Biology at HMS and the Vascular Biology Program at Boston Children's Hospital, and Professor of Bioengineering at SEAS.
More information: Weixia Zhang et al, Controllable Fabrication of Inhomogeneous Microcapsules for Triggered Release by Osmotic Pressure, Small (2019). DOI: 10.1002/smll.201903087
Journal information: Small
Provided by Harvard University