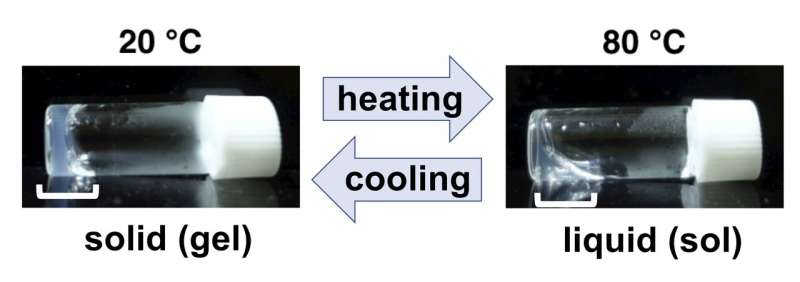
Temperature response of the peptide. It forms solid (gel) at 20 degree Celsius and liquid (sol) at 80 degree Celsius indicated by white brackets, and this feature is reversible. Credit: Takahiro Muraoka, TUAT
A collaboration mainly led by scientists from Tokyo University of Agriculture and Technology (TUAT) in Japan has developed a new method of molecular design to control both temperature reversibility and stiffness of nanofibers that are gel-forming peptides. The peptide nanofiber hydrogel can be used as biomedical material. This method will allow the peptide nanofibers for more biomedical applicable.
The researchers published their results on July 8th in Chemistry-A European Journal, which was highlighted on the Front Cover and Cover Profile.
In general, some peptides form nanofiber hydrogels. These peptides are short chains of natural amino acids found in all living organisms. Since these are bio-friendly, they have been widely used in medicine such as tissue recovery materials, regenerative medical materials, extracellular matrices, cell culture materials, and drug delivery containers.
"For some medical applications of nanofiber peptides, we need to develop a technique to control both stiffness (mechanical strength) and temperature response changing between gel (solid) and sol (liquid)," said Takahiro Muraoka, Ph.D., corresponding author on the paper and associate professor in the Department of Applied Chemistry, Graduate School of Engineering at TUAT. "It is, however, difficult to make better the both features at the same time. For example, when increasing stiffness of a peptide nanofiber by replacement of a simple amino acid alanine to a more hydrophobic amino acid phenylalanine, it is known that temperature response is often lost."
In their experiments, they found that an amino acid replacement that was thought to make a softer gel unexpectedly formed a harder gel. They used 5 sets of different peptides that had 16 amino acids. Interestingly, one particular peptide did not lose temperature response. The peptide (concentration at 1% in solution) formed gel (solid) at 20°C (68°F) and when increasing temperature to 80°C (178°F) the gel became soft (liquid). When reducing temperature from 80°C to 20°C, the solid gel was again formed. "This temperature reversible feature is applicable for drug delivery by local injection," said Muraoka.
They replaced alanine in the middle of the peptide to glycine, the simplest amino acid. The glycine replacement usually makes the gel softer. They used regular analytical instrumentation such as CD, IR, and TEM microscopy to understand precisely how the gel was formed. They also used a computational approach, called molecular dynamics simulation. "Based on our results, we are now able to design peptides better by computer simulation," said Muraoka.
Furthermore, the peptide nanofiber was cell adhesive, which is suitable as a biomaterial for cell culture and tissue regeneration. "This research will open new avenues towards designing peptide nanofibers that are more biomedical applicable," Muraoka added.
More information: Atsuya Ishida et al, Glycine Substitution Effects on the Supramolecular Morphology and Rigidity of Cell‐Adhesive Amphiphilic Peptides, Chemistry – A European Journal (2019). DOI: 10.1002/chem.201902083
Journal information: Chemistry – A European Journal
Provided by Tokyo University of Agriculture and Technology