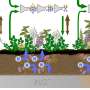
The River Thames catchment was the subject of the CEH-led modelling study. Credit: Mike Hutchins
The amount of antibiotics entering the River Thames would need to be cut by as much as 80 per cent to avoid the development and spread of antibiotic-resistant 'superbugs', a new study has shown.
Scientists from the Centre for Ecology & Hydrology (CEH) modelled the effects of antibiotic prescriptions on the development of antibiotic-resistant bacteria in a river. It found that across three-quarters of the River Thames catchment, the antibiotics present, due to effluent discharge, were likely to be at levels high enough for antibiotic-resistant bacteria to develop.
The study comes after England's chief medical officer Professor Dame Sally Davies warned last week that bugs resistant to antibiotics could pose a more immediate risk to humanity than climate change, and may kill at least 10 million people a year across the world.
Dr. Andrew Singer of the Centre for Ecology & Hydrology, who led the study, says: "Rivers are a 'reservoir' for antibiotic-resistant bacteria which can quickly spread to people via water, soil, air, food and animals. Our beaches offer a similar risk. It has been shown that surfers are four times more likely to carry drug-resistant bacteria than non-surfers."
How antibiotics get into our rivers
Up to 90% of prescribed antibiotics taken by people pass through the body and into the sewerage system, where about half end up in rivers when effluent is discharged.
Dr. Singer explains: "The release of drugs and bugs into our rivers increases the likelihood of antibiotic-resistant genes being shared, either through mutation or 'bacterial sex'. This is the first step towards the development of superbugs as the drugs used to fight them will no longer work.
"Environmental pollution from drugs and bugs is a serious problem that we need to find solutions to."
The CEH-led research was based on prescription data from clinical commissioning groups for two classes of antibiotics—macrolides, such as erythromycin and azithromycin, and fluoroquinolones such as ciprofloxacin, levofloxacin and moxifloxacin.
Macrolides treat a range of respiratory and sexually transmitted infections such as pneumonia, whooping cough and chlamydia, while fluoroquinolones treat respiratory and urinary tract infections. These were chosen for the study because they biodegrade slowly while other antibiotics such as penicillin biodegrade before they get to the river.
Possible solutions
There are a number of different ways we could reduce the amount of antibiotics entering rivers, including:
- reducing inappropriate prescriptions1, either because the antibiotics will not reduce the infection, or the course of treatment is longer than is medically necessary
- preventative action so fewer medicines are needed in the first place, such as more rapid diagnosis of medical conditions, greater uptake of vaccinations for illnesses and better hygiene controls in hospitals.
- increased investment in research and development of new wastewater treatment processes that would remove the drugs and bugs from sewage.
The Government and health officials recognise that reducing antibiotic prescribing is key to tackling antibiotic resistance, and there was a 6.1 per cent reduction in total antibiotic consumption in primary and secondary care in England between 2014 and 2018 (2).
However, antibiotics prescriptions per person in the UK per person is still higher than several European countries and double that of the Netherlands, where their controls on prescribing antibiotics and effective hygiene measures in the healthcare system have resulted in relatively low rates of antibiotic resistance.Thames Water recognises that antibiotic resistance in water is an emerging risk and that more work needs to be done on how best to manage it (3).
The study—Translating antibiotic prescribing into antibiotic resistance in the environment: a hazard characterisation case study—which also involved Royal Holloway, University of London, and received funding from UKRI, has been published in the journal PLOS ONE.
More information: Andrew C. Singer et al, Translating antibiotic prescribing into antibiotic resistance in the environment: A hazard characterisation case study, PLOS ONE (2019). DOI: 10.1371/journal.pone.0221568
Journal information: PLoS ONE
Provided by Centre for Ecology & Hydrology