August 2, 2019 feature
Two-dimensional (2-D) nuclear magnetic resonance (NMR) spectroscopy with a microfluidic diamond quantum sensor
Quantum sensors based on nitrogen-vacancy (NV) centers in diamond are a promising detection mode for nuclear magnetic resonance spectroscopy due to their micron-scale detection volume and noninductive-based sample detection requirements. A challenge that exists is to sufficiently realize high spectral resolution coupled with concentration sensitivity for multidimensional NMR analysis of picolitre sample volumes. In a new report now on Science Advances, Janis Smits and an interdisciplinary research team in the departments of High Technology Materials, Physics and Astronomy in the U.S. and Latvia addressed the challenge by spatially separating the polarization and detection phases of the experiment in a microfluidic platform.
They realized a spectral resolution of 0.65±0.05 Hz, an order-of-magnitude improvement compared with previous diamond NMR studies. Using the platform, they performed 2-D correlation spectroscopy of liquid analytes with an effective detection volume of ~40 picoliters. The research team used diamond quantum sensors as in-line microfluidic NMR detectors in a major step forward for applications in mass-limited chemical analysis and single-cell biology.
Nuclear magnetic resonance (NMR) spectroscopy is a powerful and well-established technique for compositional, structural and functional analysis in a variety of scientific disciplines. In conventional NMR spectrometry the signal-to-noise ratio (SNR) is strongly dependent on the external field strength (B0). As the spectral resolution increased, the B0 increased as well, motivating the development of increasingly large and expensive superconducting magnets for improved resolution and SNR, resulting in a two-fold increase in field strength within the past 25 years.
However, even with large B0 values, the detection of microscale volumes often required isotopic labeling, concentrated samples and long experimental timelines. To improve the sensitivity for small sample volumes, researchers developed miniature inductive coils, which allowed several advances, including spectroscopy of egg cells and in vitro diagnostics. The existing sensitivity and detection limits are yet suboptimal for metabolic analysis of single mammalian cells or for inclusion into in-line microfluidic assays. As an alternative NMR detection strategy, quantum-sensors based on nitrogen-vacancy (NV) centers in diamond have emerged due to their sub-micrometer spatial resolution and noninductive-based detection.
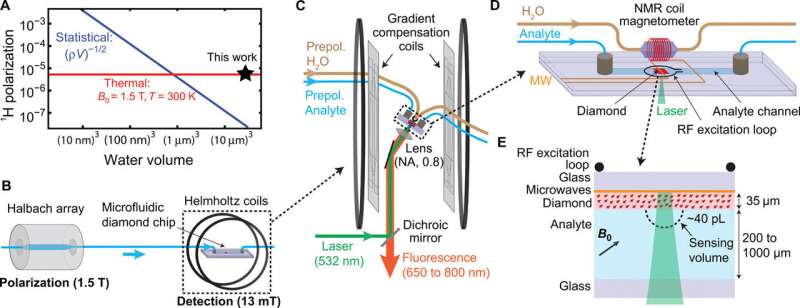
Scientists previously implemented the method to detect early nanoscale fluctuations of nuclear magnetization to enhance polarization. They used viscous solvents to improve the frequency resolution to ~ 100 Hz to obtain the resolution of large chemical shifts at B0 = 3 T (Tesla). Although further improvements in resolution can be made by increasing the detection volume (V), they were at a cost to SNR. In the present work, Smits et al. report an order-of-magnitude improvement in spectral resolution to realize a concentration sensitivity of ~ 27 M s1/2. To achieve this, they spatially separated the polarization and detection phases of the experiment in a microfluidic setup.
The research team used strong permanent magnets (1.5 Tesla) to generate nuclear spin polarization and performed the detection at 13 mT using Helmholtz coils to simplify the task of stabilizing NMR linewidths to sub-hertz levels. They facilitated the use of diamond quantum sensors as in-line microfluidic NMR detectors at low microwave frequencies. The improvements allowed Smits et al. to perform two-dimensional (2-D) correlation spectroscopy (COSY) of liquid analytes within an effective detection volume of ~ 40 pL (pico-liters). The researchers intend to combine this platform with advances in dynamic nuclear polarization using external polarizing agents and potentially optical hyperpolarization with NV centers to allow NMR spectroscopy of metabolites at physiological concentrations at single-cell spatial resolution.
In the experimental setup, Smits et al. housed the fluid analytes in a helium-pressurized container with variable flow rates of up to 50 µl/s. The dwelling time for the analytes approximated 6s, longer than the spin relaxation time of the analytes studied (for example T1 for water ≈ 3s) leading to an equilibrium polarization of ~ 5x10-6. The analyte then flowed to a detection region for identification by NV NMR. To conduct NV NMR detection, the scientists used a custom-built epifluorescence microscope and oriented diamond membranes fabricated in the study, on four possible NV axes to align with the magnetic field in the setup.
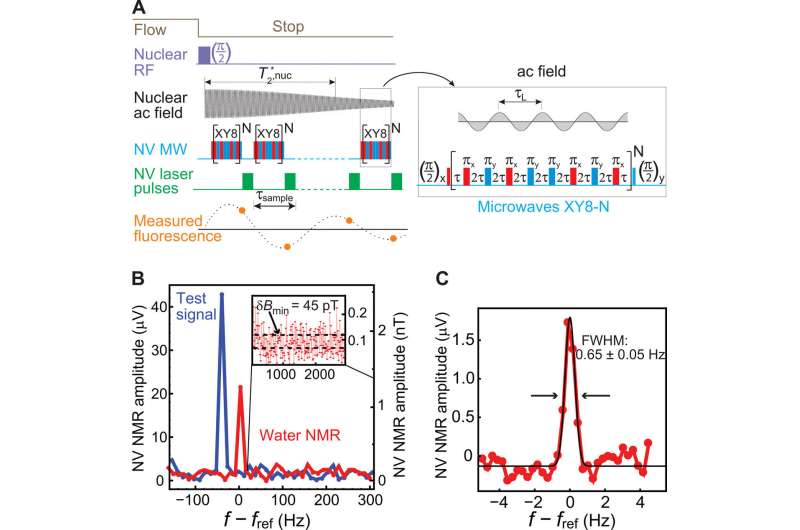
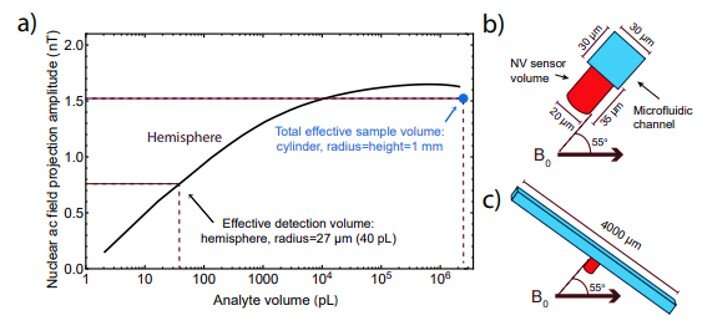
Smits et al. fabricated the microfluidic chip to house the diamond sensor, the device contained a copper chip on a glass slide to deliver the microwaves. The scientists also included a radio frequency (RF) excitation loop between the diamond and the feedback NMR coil, and a microfluidic channel enclosing the diamond sensor and the contacting analyte. They engineered microfluidic ports to combine external analyte tubing within the chip and used a 20 µm diameter laser beam to excite the NV centers through a 35 µm-thick diamond membrane.
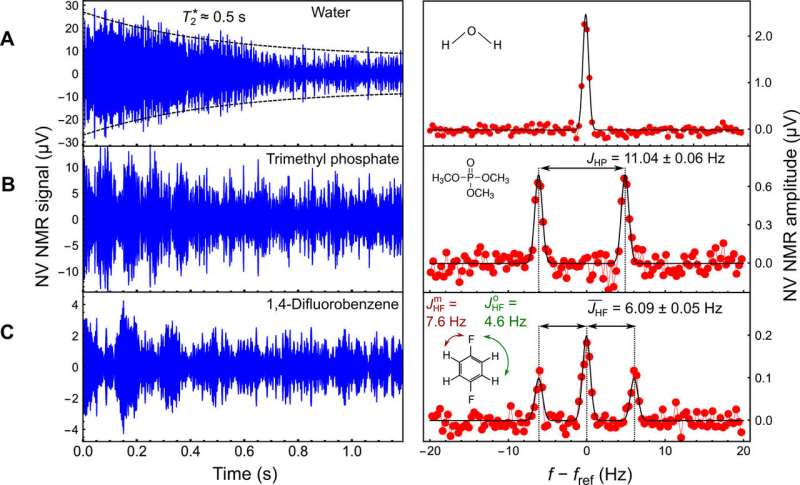
The research team subsequently applied a series of XY8-5 microwave pulse sequences to the NV center to detect the nuclear ac field. They used deionized water to determine the sensitivity and spectral resolution limits of the apparatus. To optimize the spectral resolution, they adjusted the gradient compensation coils and demonstrated the capabilities of the NV NMR spectrometer by obtaining proton NMR spectra of diverse fluid analytes.
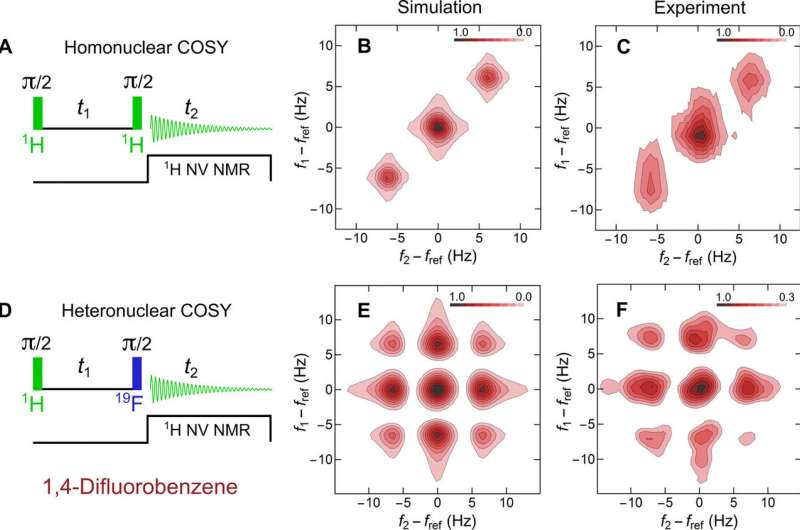
For example, the scientists obtained characteristic NV NMR spectra for trimethyl phosphate (TMP) and 1,4-difluorobenzene (DFB) compounds in the study. After establishing the potential to detect NMR spectra with sub-hertz resolution and high signal-to-noise ratios (SNRs) for the two compounds, they used the platform to perform 2-D COSY NMR spectroscopy. For this, Smits et al. performed two variants of the 2-D COSY analysis to probe nuclear interactions within DFB (1,4-difluorobenzene) and performed all simulations using the SPINACH software package for 2-D NMR.
The demonstrated sub-hertz resolution and multidimensional NMR techniques can pave the way for the use of diamond quantum sensors within in-line hyphenated analysis, single-cell metabolomics and mass-limited pharmacodynamics. Smits et al. aim for the resulting high spatial resolution and epifluorescence format to facilitate high-throughput chemical analysis and NMR imaging of cell cultures with single-cell resolution. Limitations of the present device include the substantial averaging times required at physiological concentrations ranging from the micromolar to the millimolar volume. The researchers propose to use higher magnetic fields with longer and more sensitive XY8-N microwave pulse sequences to improve NMR sensitivity and photon collection efficiency in contrast to the existing methods. In the long-term, they expect the largest gains in sensitivity to occur via non-invasive optical hyperpolarization methods.
The use of low external field strength (B0) of 13 mT was another limitation in the study since it restricted the ability to resolve spectral splitting due to chemical shifts. The team aims to improve the chemical shift resolution by increasing B0 to ~ 0.25 T, using the present detection scheme. In addition, although the NMR microfluidic sensor had an effective detection volume of ~40 pL, the scientists required several milliliters of analyte to fill the overall flow of the apparatus. Future microfluidic chips can therefore either miniaturize or omit pre-polarization steps or use smaller microfluidic channels for detection in a larger fluidic system.
In this way, Janis Smits and co-workers demonstrated the use of diamond quantum sensors for microfluidic NMR applications. They showed that separating the polarization and detection steps allowed an order-of-magnitude improvement in spectral resolution compared with existing diamond NMR studies. The scientists validated the platform by performing 2-D NMR on fluid analytes and propose its future applications in multidisciplinary research fields.
More information:
1. Janis Smits et al. Two-dimensional nuclear magnetic resonance spectroscopy with a microfluidic diamond quantum sensor, Science Advances (2019). DOI: 10.1126/sciadv.aaw7895
2. R. Zenobi. Single-Cell Metabolomics: Analytical and Biological Perspectives, Science (2013). DOI: 10.1126/science.1243259
3. P. Kehayias et al. Solution nuclear magnetic resonance spectroscopy on a nanostructured diamond chip, Nature Communications (2017). DOI: 10.1038/s41467-017-00266-4
4. David R. Glenn et al. High-resolution magnetic resonance spectroscopy using a solid-state spin sensor, Nature (2018). DOI: 10.1038/nature25781
Journal information: Science Advances , Science , Nature Communications , Nature
© 2019 Science X Network