Credit: CC0 Public Domain
A team of researchers with the University of Nottingham, Jealott's Hill International Research Centre and the GlaxoSmithKline, Medicines Research Centre, has found a way to use a phosphine oxide catalyst to make nucleophilic substitution reactions of alcohols greener. In their paper published in the journal Science, the group describes their process and its benefits. Lars Longwitz and Thomas Werner with the Leibniz Institute for Catalysis have published a Perspective piece describing the work done by the team in the same journal issue.
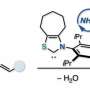
Nucleophilic substitutions are a type of substitution reaction used in organic chemistry—they involve using a nucleophile to replace a good leaving group. Such reactions (Mitsunobu chemistry) are commonly used as part of a process to synthesize a wide variety of commercial products. Longwitz and Werner note that alcohols are typically used as the nucleophile because they are inexpensive and easily obtainable. But because alcohols do not react with pronucleophiles unless they are active prior to the substitution, the reaction leads to the production of an undesirable phosphine oxide co-product—a less-than-clean outcome. In this new effort, the researchers have come up with a way to avoid this problem, and thus provide a much cleaner method of inverting the configuration of alcohols.
The method developed by the researchers was eight years in the making and involved using a phosphine oxide the team designed that does not require reductants or oxidants. The technique does not require an azo reagent. To activate the phosphine oxide catalyst, they used acidic protons that were meant for the nucleophile used in the substitution. Next, they dehydrated the catalyst, forcing it into a ring-shaped structure. Doing so cut the phosphorus-oxygen double bonds, which, in past efforts, has proven to be quite difficult. Doing so allowed oxygen molecules to attach to the phosphorus atoms, forcing the ring open—and that allowed the coupling partner to attack the resulting salt, which produced the end product. The process not only produces only water as a byproduct, but also regenerates the catalyst. Longwitz and Werner suggest the method treads a new path toward more sustainable organic synthesis.
More information: Rhydian H. Beddoe et al. Redox-neutral organocatalytic Mitsunobu reactions, Science (2019). DOI: 10.1126/science.aax3353
Journal information: Science
© 2019 Science X Network