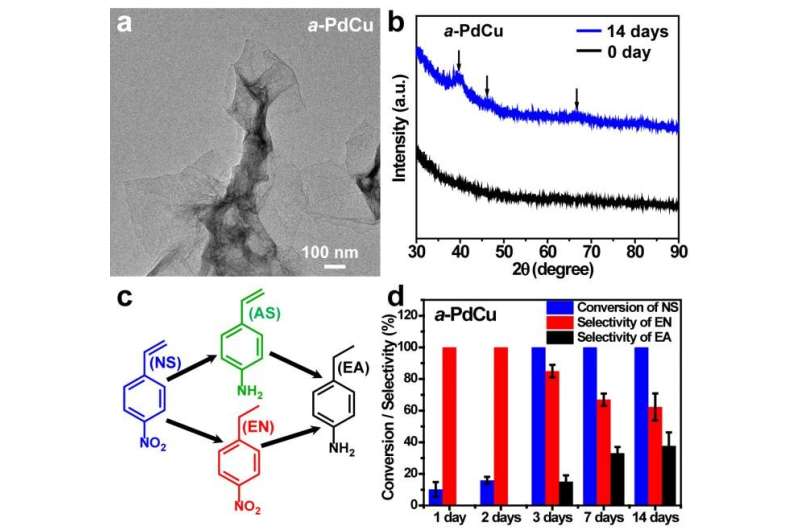
(a) TEM image of a-PdCu nanosheets. (b) XRD patterns of the as-synthesized (0 day) a-PdCu and the a-PdCu after aging for 14 days. (c) Hydrogenation reaction of 4-nitrostyrene (room temperature, H2 balloon). (d) The catalytic results of 30-min hydrogenation reaction using a-PdCu with different aging time as catalysts. Credit: ©Science China Press
Selective catalysis plays a key role in various applications, such as the chemical industry and oil refining, hence, developing catalysts with high efficiency and excellent chemoselectivity has become a research hotspot. Compared with other materials, noble metals, especially ultrathin two-dimensional (2-D) noble metal nanomaterials, have drawn tremendous research interest owing to their superior catalytic activity in many catalytic reactions.
In recent years, phase engineering is emerging as a promising and challenging research field in noble metal-based nanomaterials. In particular, as one kind of heterophase nanostructures—amorphous/crystalline heterophase nanostructures—have been prepared and have demonstrated promising catalytic performance. The random atomic arrangement in amorphous phase results in highly unsaturated coordination and abundant active sites for catalytic applications. In addition, the amorphous/crystalline interfaces can also benefit catalytic activity.
However so far, it is still very challenging for wet-chemical synthesis of ultrathin 2-D noble metal-based nanoalloys with an amorphous/crystalline heterophase. The thermodynamically stable phases for noble metals are close-packed crystalline structures due to the strong atomic interaction, thus it is thermodynamically unfavored to form an amorphous phase, which has random atomic arrangement. In addition, the atomic isotropy of the amorphous phase also makes it very challenging to obtain the 2-D structure.
Herein, Prof Zhang Hua's group has prepared two types of amorphous/crystalline heterophase PdCu nanosheets, of which one is amorphous phase-dominant (a-PdCu) and the other one is crystalline phase-dominant (c-PdCu). Since amorphous phase in metals tends to transform into crystalline phase under ambient conditions, the phase transformation behavior of the synthesized heterophase PdCu nanosheets and the heterophase-dependent properties have been systematically studied. During the aging process, the crystallinity of a-PdCu gradually increased, which was accompanied with changes in some other physicochemical properties, including electronic binding energy and surface ligand adsorption. Consequently, the chemoselectivity and catalytic activity of the heterophase PdCu nanosheets also changed in the hydrogenation of 4-nitrostyrene.
It was found that in the first 2 days of the aging process, the a-PdCu showed very high chemoselectivity, while the c-PdCu did not show chemoselectivity. After 3-day aging, the a-PdCu lost the chemoselectivity, but its catalytic activity gradually increased, while the catalytic activity of c-PdCu gradually decreased and eventually became lower than that of the a-PdCu after aging for 14 days. This work demonstrates the intriguing properties of heterophase nanostructures, providing a new platform for future studies on the regulation of functionalities and applications of nanomaterials by phase engineering.
More information: Hongfei Cheng et al, Aging amorphous/crystalline heterophase PdCu nanosheets for catalytic reactions, National Science Review (2019). DOI: 10.1093/nsr/nwz078
Provided by Science China Press