Third-generation sequencing (TGS) technologies like the portable MinION sequencer promise to revolutionize biology, but getting there will require tweaking techniques. Particularly, the low output delivered by TGS sequencers means that targeted sequencing approaches will have to be developed to assure proper sequencing coverage of regions of interest. In research presented in a recent issue of Applications in Plant Sciences, Dr. Thomas Couvreur and colleagues developed a new protocol to capture and sequence longer segments of plastome DNA in plants.
"New sequencing technologies like the MinION can play a pivotal role in accelerating biodiversity discovery and understanding its functioning and evolution, especially in the tropics. This is because sequencing is becoming more portable and easy to do," said Dr. Couvreur, corresponding author on the article and Director of Research at the French National Institute for Research for Sustainable Development (IRD) in Montpellier, France. "We were thus interested in improving our lab protocols to better integrate these technologies for biodiversity research."
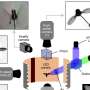
In particular, Dr. Couvreur and colleagues were interested in capturing and sequencing long fragments of chloroplast DNA using a technique called targeted sequencing, which had never been used for long DNA fragments in plants. They designed probes to capture these long sequences and tested the protocol in six monocots—three grasses and three palms. Dr. Couvreur describes the targeted capture as "a bit like fishing. We basically hook the DNA we are interested in and then sequence it."
Targeted sequencing combined with TGS technology could improve genome assembly, or the computational stitching together of many sequenced DNA fragments into larger continuous blocks. The short reads produced by older sequencing technologies, typically 100-400 base pairs long, make bioinformatic assembly of certain genomic regions like repetitive sequences very difficult. Third-generation sequencing technologies such as the portable MinION produce longer reads that could help produce these assemblies. However, these sequencers generally have lower data output than older sequencing technologies. Therefore, to efficiently sequence regions of interest, these regions must be enriched through methods like targeted capture and sequencing.
"Longer fragments makes assembling genomes easier. One can compare this to a puzzle with 1000 small pieces (short reads) versus 10 large pieces," explained Dr. Couvreur. "Our research question was simple: can we capture long DNA fragments for targeted sequencing?" The team did succeed in capturing long fragments of plastome DNA. The median fragment length was 3151 base pairs long on average across trials, much longer than the ~400 base pair fragments delivered by older sequencing technologies.
"This method can be used to assemble plastomes, or at least significantly improve assembly," said Dr. Couvreur. "Our protocol will be particularly useful for non-model plant species, for which we have little or no prior data on the plastome." This can help in phylogenetic and other analyses of non-model plants, which represent the vast majority of plant biodiversity.
"The field of targeted sequencing is changing quickly, and improved protocols will no doubt enhance plastome assembly," concluded Dr. Couvreur. "For now, we show that capturing and subsequently sequencing long DNA fragments is possible, which is a first step."
More information: Kevin Bethune et al, Long‐fragment targeted capture for long‐read sequencing of plastomes, Applications in Plant Sciences (2019). DOI: 10.1002/aps3.1243
Journal information: Applications in Plant Sciences
Provided by Botanical Society of America