Bacteria-attacking phages could provide clues to antibiotic resistance
Is there a solution to bacteria becoming resistant to antibiotics? One answer may be found by studying the world's largest and most brutal army, new University of Otago microbiology research shows.
In fact that army—bacteria-attacking viruses known as phages—outnumber bacteria nearly ten-fold, making them the most abundant biological entity on earth. They are intricately adapted to invading, weakening and controlling their targets, then keeping them alive long enough to feed off them and use them to breed.
To defend themselves from the phage invasion, bacteria have developed "CRISPR" defense systems—immune systems within the bacteria. But the phages have their own weapon, called "anti-CRISPR," which blocks these bacterial defenses.
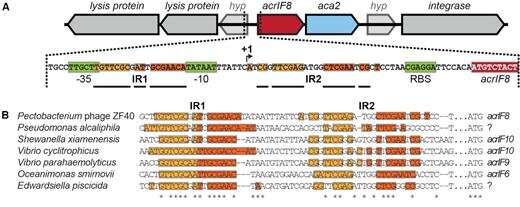
The Otago study's lead author, Ph.D. student Nils Birkholz, of the University's Department of Microbiology and Immunology, says the new research shows phages use a special protein to control the production of the anti-CRISPR when they invade a bacteria.
Initially they rapidly ramp up anti-CRISPR production, conquering the bacteria's defense system. Then, with the bacteria beaten, the phages use the protein to switch off anti-CRISPR production, ensuring the bacteria survives—conquered, but alive.
That makes sense, Mr Birkholz says, as phages, like all viruses, are not just conquerors but hijackers that reproduce inside living hosts.
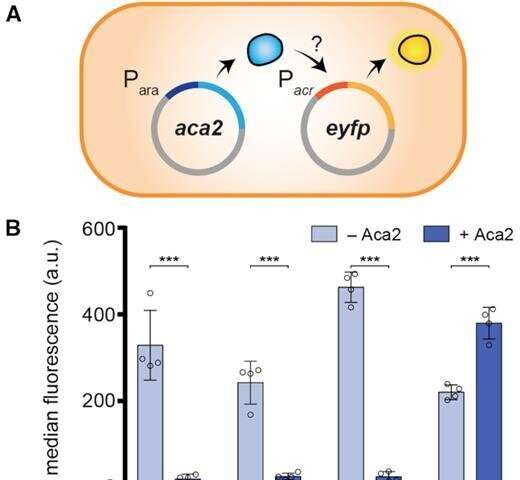
"We know from previous research that too much anti-CRISPR production can be bad for the cell and an important question was how anti-CRISPR abundance is controlled. This has now been answered by our research.
"Our results suggest that right after infection, the phage produces a large enough anti-CRISPR quantity to inhibit bacterial defense, but then turns down production to avoid any negative side effects.
"So this protein ensures that, once the virus has beaten its host it keeps it alive and devotes its resources to its own reproduction."
That is, until the phages finish reproducing, at which point they explode out of the cells, killing them, before moving on to infect other cells.
The results emphasize the "delicate balancing act" phages need to perform to subdue their hosts, Mr Birkholz says. And as gruesome as it sounds, the research could lead to very "real world" outcomes.
"Phages have a strong impact on our lives in both negative and positive ways. Particularly in this era of increasing antibiotic resistance, phages are being considered as a means to treat bacterial infections. So these new details provide information that might help us choose, or design, more effective antimicrobial phages."
The research paper, called "The autoregulator Aca2 mediates anti-CRISPR repression" and published in Nucleic Acids Research this month, was written by Nils Birkholz, Robert D. Fagerlund, Leah M. Smith, Simon A. Jackson and Peter C. Fineran.
More information: Nils Birkholz et al. The autoregulator Aca2 mediates anti-CRISPR repression, Nucleic Acids Research (2019). DOI: 10.1093/nar/gkz721
Journal information: Nucleic Acids Research
Provided by University of Otago