Credit: CC0 Public Domain
A Netherlands Cancer Institute team, co-led by Thijn Brummelkamp and Anastassis (Tassos) Perrakis, reported independently, but almost simultaneously with three more groups from all over the world, on the crystal structure and mechanism of a peculiar molecular end-tail of the microtubules that constitute the cell skeleton.
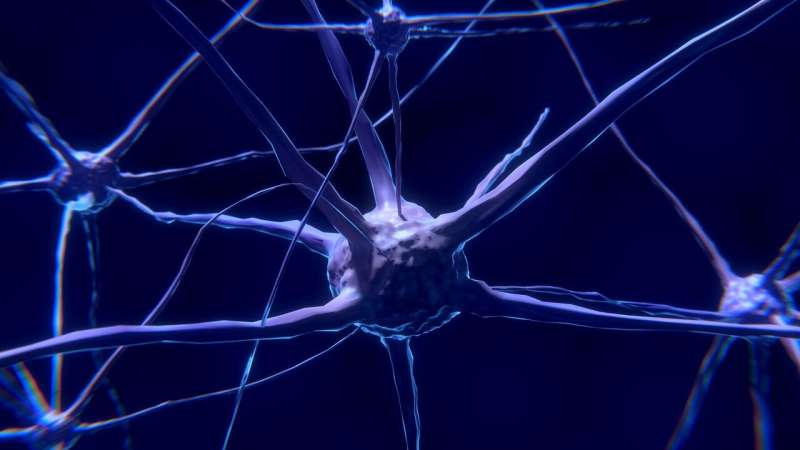
A cell skeleton is made of cables called microtubules. These allow a cell to maintain its shape, move to different places and transport molecules through its interior. Microtubules also play a key role in cell division.
The frequently used cancer therapeutic paclitaxel, aimed at cells that are dividing, specifically acts on microtubules and thereby affects their detyrosination. In addition, detyrosynation of tubulin has been implicated in cardiac dysfunction, correct segregation of chromosomes during mitosis, and mental retardation.
Microtubules are continuously modified to serve different purposes within the cell. For this, their tyrosine tails are cut and put back by different enzymes. After researchers from the Netherlands Cancer Institute and Oncode Institute, in 2017, found the identity of the scissors that remove the tail, an apparent race was launched to solve the next piece of the puzzle: to determine the three-dimensional structure of these molecular scissors.
This month, the Netherlands Cancer Institute team, co-led by Thijn Brummelkamp and Anastassis (Tassos) Perrakis, simultaneously with three groups from all over the world, are reporting on the crystal structure and mechanism of these peculiar molecular end-tail scissors. Tassos Perrakis says, "This means that a beautiful consensus is emerging, supported by complementary experiments which together have been constructing an exciting story."
More information: Athanassios Adamopoulos et al. Crystal structure of the tubulin tyrosine carboxypeptidase complex VASH1–SVBP, Nature Structural & Molecular Biology (2019). DOI: 10.1038/s41594-019-0254-6
Journal information: Nature Structural & Molecular Biology
Provided by Netherlands Cancer Institute