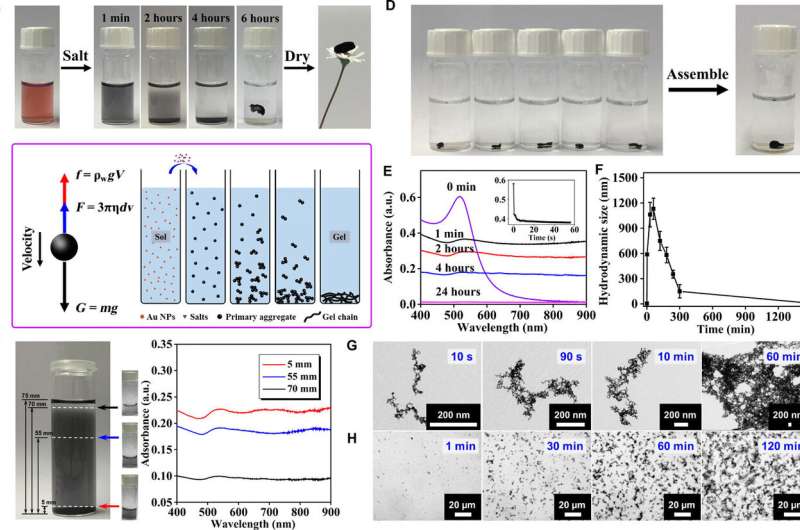
Analysis of the overall gelation process of gold NPs. (A) Digital photos of the gel preparation process. (B) Schematic demonstration of the gelation process and a corresponding force analysis. (C) The gradient distribution during the gelation characterized by ultraviolet-visible (UV-vis) absorption spectra. a.u., arbitrary units. (D) Several pieces of as-prepared hydrogels can assemble to one piece. (E to H) Time-lapse (E) UV-vis absorption spectra, (F) hydrodynamic size, (G) transmission electron microscopy (TEM), and (H) optical microscopy characterization during gelation. The inset in (E) shows the time-lapse UV-vis absorption evolution at 510 nm, which was recorded during the first minute upon reaction. (Photo credit: Ran Du.) Credit: Science Advances, doi: 10.1126/sciadv.aaw4590
Noble metal foams (NMFs) are a new class of functional materials that contain both noble metals and monolithic porous materials for impressive multi prospects in materials science and multidisciplinary fields. In a recent study now published on Science Advances, Ran Du and a team of interdisciplinary researchers in Physical Chemistry, Materials Engineering and Physics developed highly tunable NMFs by activating specific ion effects to produce a variety of single/alloy aerogels. The new materials contained adjustable composition—with gold (Au), silver (Ag), palladium (Pd) and platinum (Pt)—and special morphologies.
The NMFs exhibited superior performance as programmable self-propulsion devices, which the scientists proved using electrocatalytic alcohol oxidation reactions. The study provided a conceptually new approach to engineer and manipulate NMFs to provide an overall framework and understand the mechanisms of gelation. The work will pave the way forward to design on-target NMFs to investigate structural performance relationships for a variety of applications.
Functional porous materials are an interesting topic at the cutting edge of materials science, combining porous structures and versatile compositions for multidisciplinary applications. Noble metal foams (NMFs) are a rising star in the foam family and have gained tremendous attention during their debut. The addition of noble metals into 3-D gel networks has enhanced NMFs with a variety of potential applications but their development is still in the early stages with limited fabrication strategies and lesser understood structural properties that cannot be well manipulated.
Typically, NMFs are engineered using four classes of methods, which include:
Of these, the sol-gel process has substantially produced nanostructured and high surface areas for NMFs under mild conditions to become a popular synthetic strategy. Nevertheless, the sol-gel process is at an infant stage with numerous mysteries surrounding the process; constraining its exploration to understand gelation mechanisms for on-demand manipulation.
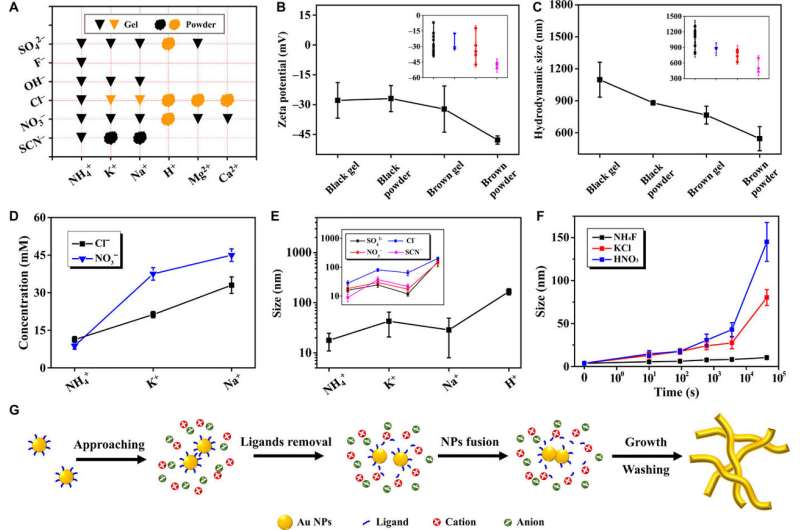
Analysis of the specific ion effects on the gelation behavior and ligament size. (A) Summary of the status of gels induced by different ions. The inverted triangle and the diffused circle indicated the gel and powder, and black and brown indicated the color of the products. (B) Zeta potential upon reaction and (C) dh versus the color and form of products. The data were obtained by averaging detailed values from the inset diagram. (D) The low-threshold gelation concentration of salts (cs) versus the used cations. (E) The ligament size (averaged over the anions used as in the inset diagram) of as-synthesized gold aggregates versus cations. (F) Time-lapse ligament size evolution of gold aggregates induced by three typical salts. (G) Proposed mechanism for gel formation. Credit: Science Advances, doi: 10.1126/sciadv.aaw4590
In the present work, Du et al. presented a method for rapid fabrication and flexible manipulation of NMFs by activating and designing specific ion effects. For this, they experimentally studied in depth gelation processes alongside complementary DFT calculations to outline the overall reaction process. Du et al. realized versatile compositions with multiple alloys, ligament sizes, specific surface areas and spatial element distribution during materials synthesis. The method and the enormous ion library developed in the work will offer unprecedented opportunities to manipulate NMFs and extend to diverse colloidal solution systems, as demonstrated with electrocatalytic alcohol oxidation and a dark-to-shining chemical reaction.
Du et al. first added the gold nanoparticles (NP) solution with specific salts and grounded it from 4 to 12 hours to yield the hydrogel, then freeze-dried it further to obtain the corresponding aerogel. The NMFs indicated a robust gelation capacity and completely eliminated the need for expensive concentration processes. The approach used by the scientists uniquely allowed for rapid gelation of the metal precursors at low concentrations and ambient temperature.
Demonstration of black gels, brown gels, and black powders as prepared in the study.Credit: Science Advances, doi: 10.1126/sciadv.aaw4590
To explain the unconventional phenomenon, they proposed a gravity-driven assembly model where the salt-initiated aggregates grew gradually and settled down due to gravity to concentrate and evolve into a hydrogel at the bottom. The scientists supported this model using UV-VIS absorption spectra to visualize the entire process of gelation. Since hydrogels can self-repair, the materials displayed promising self-healing properties in diverse environments without external energy input.
Du et al. conducted time-lapse characterization studies to test the extremely fast formation of aggregates with multiscale microstructures. Additionally, they competed time-lapse transmission electron microscopy (TEM) and in situ optical tests to reveal the evolutionary footprints of 3-D networks at different scales. Using the analytical techniques, the scientists observed the formation of gold nanoparticle (NP) dimers, followed by their gradual axial growth to form nanowire-structured networks during the sol-gel process of NMF fabrication.
The scientists showed how the experimental outcomes varied the form (gel to powder) and color (black to brown) of the ions, to strongly correlate to the salting-out effects as dictated by the Hofmeister series (a classification of ions according to their ability to salt-out or salt-in proteins). They used time-lapse TEM imaging to further reveal the growth mode of NPs and the ligament size variation during network development and proposed a possible mechanism during NMF formation via the sol-gel process. Accordingly;
- The original NPs approached each other instantly on the addition of salts due to electrostatic screening.
- The ligands then partially stripped away from the NPs by oppositely charged cations
- Followed by the generation of NPs to form aggregates driven by the raised surface energy of uncapped NPs
- The aggregates repeated the process across axial and radial directions
- For the aggregates to finally settle by gravity-driven sedimentation to form hydrogel at the bottom.
The ability to systematically manipulate the ligament size and corresponding physical properties of NMAs was not previously realized. As a result, Du et al. deeply studied the gelation process to unlock specific ion effects and strategies of manipulation. For this, they deliberately selected specific salts (NH4SCN, NH4NO3 and KCl) as initiators.
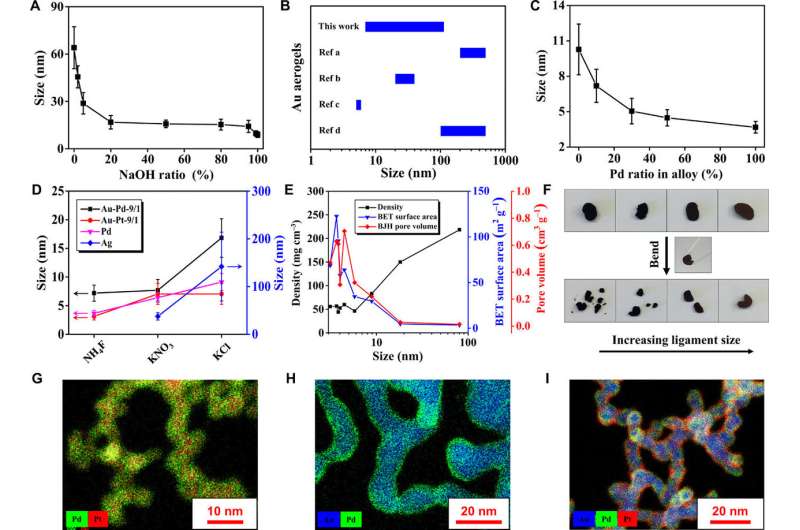
Versatile manipulation of NMAs. (A) Tailor the ligament size of gold gels by introducing NaOH/NaCl hybrid salts. (B) Ligament size of gold aerogels from different references of studies previously conducted. (C) The ligament size variation with Au/Pd ratio. (D) Ligament size modulation of Au-Pd, Au-Pt, Pd, and Ag gels using different salts. (E) The dependence of density, Brunauer-Emmett-Teller (BET) surface area, and Barrett–Joyner–Halenda (BJH) pore volume of aerogels versus ligament size. (F) Demonstration of the size-dependent mechanical properties of aerogels by bending with a tweezer. From left to right are Au-Ag-NH4F (5.8 ± 0.7 nm), Au-NH4SCN (8.9 ± 2.5 nm), Au-NH4NO3 (18.2 ± 4.0 nm), and Au-NaCl (64.0 ± 13.3 nm), respectively. (G to I) STEM–energy-dispersive x-ray spectroscopy (EDX) of three alloy gels with (G) homogeneous and (H and I) core-shell architectures. Credit: Science Advances, doi: 10.1126/sciadv.aaw4590
They observed a brown color for the KCl-induced aerogel, while two other aerogels with smaller ligament sizes appeared black due to strong light absorption / scattering between nanosized domains. Changing the ligament size also changed their density, specific surface area and pore volume. The scientists showed improved outcomes for ligament size and additional properties by using hybrid-salts in the experimental setup. Based on the proposed gelation mechanism, they expanded the system to include noble metals and their alloys (Ag, Pd, and Pt).
The present work provided set guidelines to engineer the physical parameters of NMAs. This is an important outcome since the physical and mechanical properties of NMAs currently remain a great challenge to be realized. The straightforward, synthetic approach introduced in the present work provided a variety of bimetallic and trimetallic gels with well defined, tunable core-shell architecture.
Since metals are remarkably ductile, the scientists induced a dark-to-shining transition by manually rearranging the NMAs from the millimeter to micrometer scale to regain a metallic gloss with nanostructured "mirror surfaces." Du et al. welded together different aerogels to form macroscopic heterostructures and the extraordinary plasticity of the materials allowed the scientists to arbitrarily shape and encase the NMAs within elastomers for use as flexible conductors. Using catalytic oxygen evolution they maintained the different NMAs as an alternative to the expensive Platinum-based conductors.
Demonstration of pressing original aerogels into shining materials. Credit: Science Advances, doi: 10.1126/sciadv.aaw4590
During electrocatalysis of alcohol electro-oxidation reactions, the scientists showed that Au-Pd and Au-Pd-Pt aerogels performed substantially better compared with commercial Pd/C or Pt/C catalysts. The results also showed higher performance compared to previously reported NMAs such as Pd-Cu, Pd-Ni and Au-Ag-Pd aerogels. However, the scientists recorded considerable current decay for the Au-Pd and Au-Pd-Pt aerogels during long-term tests; a common issue for commercial catalysts. The optimized electrocatalytic potential will allow aerogels to function as anodic catalysts in various fuel cells and enhance electrical conductivity to facilitate efficient electron transfer during electrocatalysis.
In this way, Du and co-workers developed a specific ion-directed gelation strategy to rapidly manufacture and flexibly manipulate NMAs at room temperature from a nanoparticle (NP) solution. Using the experimental results and DFT calculations they proposed an overall mechanism for the sol-gel process. The present work provides a new concept and straightforward approach to fabricate different NMAs. The work will pave the way forward for materials scientists to design on-target, versatile NMFs for a variety of applications using structure-performance relationships to form on-demand desirable properties.
More information: Ran Du et al. Specific ion effects directed noble metal aerogels: Versatile manipulation for electrocatalysis and beyond, Science Advances (2019). DOI: 10.1126/sciadv.aaw4590
A. G. Slater et al. Function-led design of new porous materials, Science (2015). DOI: 10.1126/science.aaa8075
S. S. KISTLER. Coherent Expanded Aerogels and Jellies, Nature (2008). DOI: 10.1038/127741a0
Journal information: Science Advances , Science , Nature
© 2019 Science X Network