Ultra-thin superlattices from gold nanoparticles for nanophotonics
Researchers led by Prof. Dr. Matthias Karg at the Institute of Physical Chemistry report a simple technique for developing highly ordered particle layers. The group worked with tiny, deformable spherical polymer beads with a hydrogel-like structure. Hydrogels are water-swollen, three-dimensional networks. Such structures are used as super-absorbers in such products as babies' nappies due to their ability to soak up large quantities of liquids.
Within these hydrogel beads are tiny gold or silver particles just a few nanometres in size, which Karg's team synthesizes at HHU using metal salts in a reduction process. "We can adjust the size of the gold particles very precisely because the hydrogel shells are permeable to dissolved metal salts, allowing for successive overgrowth of the gold cores." The structure of these core-shell particles can be roughly compared to that of a cherry, in which a hard core is surrounded by soft pulp.
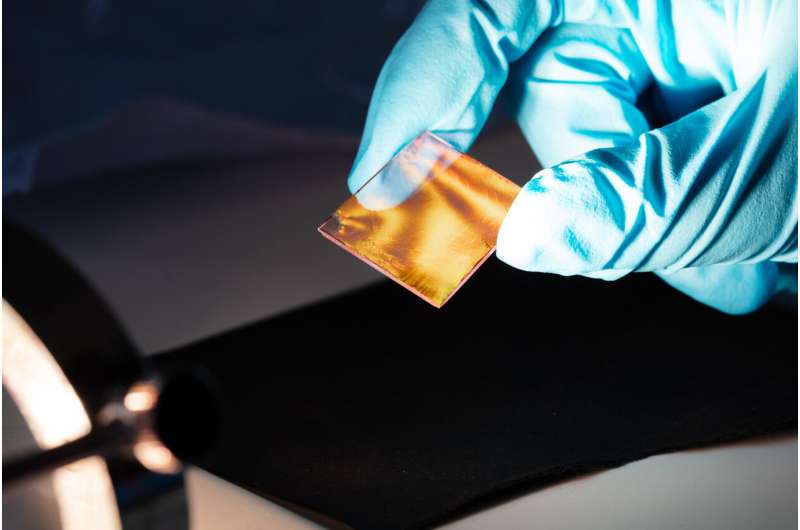
The Duesseldorf-based researchers used a dilute solution of these hydrogel beads to produce thin monolayers. They applied the beads to a water surface, where a shimmering, highly ordered layer self-assembled. The researchers transferred this layer from the water surface onto glass substrates; this transfer makes the glass substrate shimmer.
Looking at such a layer with an electron microscope reveals a regular, hexagonally ordered particle array. "These are the gold particles in their shells," explains doctoral student Kirsten Volk, "and we see that they are arranged in a single, highly ordered layer." The gold particles determine the colour of the layer by reflecting visible light with certain wavelengths, which interferes and thus creates the impression of a changing colour when viewed from different angles.

"These thin layers are very interesting for optoelectronics—i.e., the transfer and processing of data using light. It may also be possible to use them to build miniaturised lasers," says Prof. Karg. These nanolasers are only nanometres in size, thus constituting a key technology in the field of nanophotonics.
In their study published in ACS Applied Materials & Interfaces, the Duesseldorf-based researchers have overcome a major obstacle on the path to such nanolasers. They created collective resonances in the gold particles by incident light. This means that the gold particles are not excited individually; instead, all excited particles are in resonance. This collective resonance is the basic prerequisite for building lasers. The particle layers are also very thin.
For optoelectronic applications and nanolasers, the resonant modes will have to be amplified further in the thin layers. Prof. Karg says, "Next, we will try to amplify the resonance further by means of doping with emitters. In the long term, this could also allow us to realise electrically powered nanolasers."
More information: Kirsten Volk et al, In-Plane Surface Lattice and Higher Order Resonances in Self-Assembled Plasmonic Monolayers: From Substrate-Supported to Free-Standing Thin Films, ACS Applied Materials & Interfaces (2019). DOI: 10.1021/acsami.9b03197
Journal information: ACS Applied Materials and Interfaces
Provided by Heinrich-Heine University Duesseldorf


















