For decades, most industries, from petrochemicals to paper, have embraced continuous manufacturing processes. In contrast, the ultraconservative pharmaceutical industry has remained committed to batch operations. But recently, the demands of chemically complex and targeted drugs coming to market have caused many pharmaceutical companies to rethink the way they make medicines, according to an article in Chemical & Engineering News (C&EN), the weekly newsmagazine of the American Chemical Society.
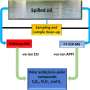
Traditionally, pharmaceutical companies made drugs by moving them through each step of the manufacturing process in single large batches. Continuous processes, on the other hand, produce drug ingredients or finished products such as tablets continuously, without having to wait for each batch to finish before beginning a new one. Advantages of continuous processes include reduced cost, factory space and processing time, as well as access to more complex chemistries. Senior Editor Rick Mullin writes that until recently, pharmaceutical companies have been wary of continuous manufacturing because of perceived perils, such as low product volume, regulatory uncertainty and commitment to current manufacturing assets.
In 2015, the U.S. Food and Drug Administration approved the first finished-dose drug—a cystic fibrosis medication called Orkambi, produced by Vertex Pharmaceuticals—made by an entirely continuous tableting process. Since then, Janssen, Eli Lilly and Company, and Pfizer have received approvals for drugs made by continuous processes. And now, companies are turning their attention to the continuous production of active pharmaceutical ingredients, which have proven more challenging than finished-dose tablets. Synthesizing the ingredients with continuous technologies, such as processes that incorporate photochemistry, cryogenic/exothermic chemistry and other techniques, offers capabilities not readily available in batch processes, experts say.
More information: "Off the drawing board," cen.acs.org/pharmaceuticals/Of … drawing-board/97/i17
Provided by American Chemical Society