Scientists develop artificial chemical receptor to assist viral transduction for T cell engineering
Engineered T cell immunotherapy, such as chimeric antigen receptor T cell (CAR-T) and T cell receptor T cell (TCR-T) therapy, has emerged as a potent therapeutic strategy for treating tumors.
However, the genetic manipulation of primary T cells remains inefficient, especially during the clinical manufacturing process. There's an urgent need to develop a reliable method for the preparation of engineered T cells.
A research team led by Prof. Cai Lintao at the Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Sciences and other collaborators developed a "safe, efficient and universal" technique based on bioorthogonal chemistry and glycol-metabolic labeling for viral-mediated engineered T cell manufacturing. Their findings were published in Advanced Functional Materials.
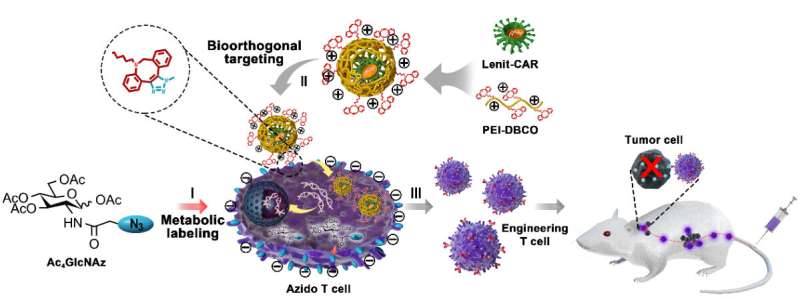
In this strategy, the functional azide motifs were anchored on T cell surfaces via the intrinsic glycometabolism of exogenous azide-glucose, thus serving as an artificial ligand for viral binding. The complementary functional moiety dibenzocyclooctyne (DBCO)/-conjugated PEI1.8K (PEI-DBCO) was coated on the lentiviral surface, which strengthened the virus-T cell interaction through DBCO/azide bioorthogonal chemistry.
"We found that this artificial chemical receptor effectively facilitated viral binding to T cells and elevated the transduction efficiency of the lentivirus from 20 percent to 80 percent without any effect on T cell proliferation and activity," said Cai. "This artificial chemical modification was also appropriate for introducing other heterologous genes into T cells, including GPF, CAR and TCR, indicating a great potency for universal T cell engineering."
The technique has been demonstrated to be safe for human primary T cells as well, without interference from cell expansion or antitumor functions. When put into the CAR-T preparation, the PEI-DBCO/azide-glucose system significantly increased the yield of CAR T cells and boosted their antitumor effect both in vitro and in the B lymphoma xenograft mouse model with a low dose of CAR-T cells, thus reducing clinical adverse effects.
"This artificial chemical labeling strategy is an effective, safe and easy upgrade for viral-based gene manipulation of human primary T cells, thereby showing great potential for clinical engineered T lymphocyte manufacturing, including CAR-T and TCR-T cell therapy," said Cai.
Prof. CaI, the corresponding author of the paper, was selected as a Fellow of the American Institute for Medical and Biological Engineering (AIMBE) on March 25 at the American Academy of Sciences in Washington, a nonprofit organization founded in 1991, for his contributions involving optical probes and biomimetic drug delivery systems in the fields of nanomedicine and cancer theranostics.
More information: Hong Pan et al, Glycometabolic Bioorthogonal Chemistry‐Guided Viral Transduction for Robust Human T Cell Engineering, Advanced Functional Materials (2019). DOI: 10.1002/adfm.201807528
Journal information: Advanced Functional Materials
Provided by Chinese Academy of Sciences