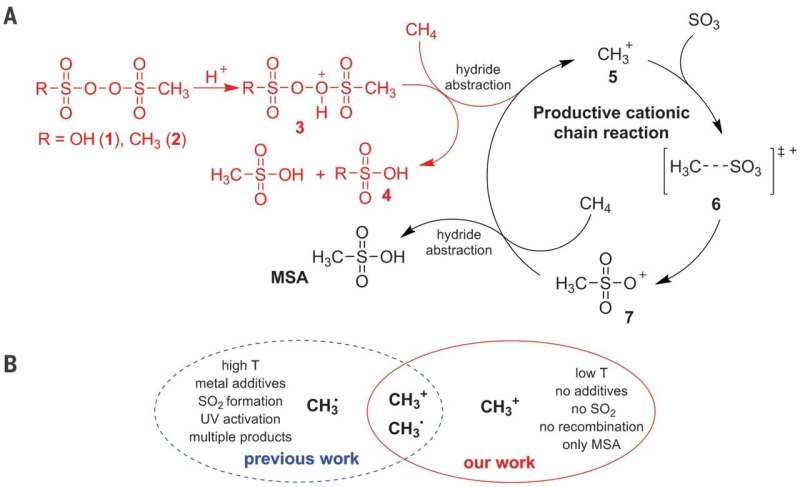
(A) Proposed ionic reaction mechanism for the C–H activation of CH4 in the selective production of MSA (Methanesulfonic acid). (B) Advantages of the cationic pathway over the radical pathway. T, temperature. Credit: Science, doi: 10.1126/science.aav0177
Methane is a major component in natural gas and one of the most difficult molecules for controlled activation, since most of the product results in carbon dioxide. The industrial conversion of methane to alcohol derivatives is typically based on a circuitous route that begins with overoxidation to carbon monoxide. Although more direct approaches have shown promise in highly acidic media at a small scale, they are not quite cost-effective. In a recent study now published in Science, Christian Díaz-Urrutia and Timo Ott at the R&D department of Grillo-Werke AG Company describe a reaction at a pilot-plant scale that directly combined methane (CH4) and sulfur trioxide (SO3) in sulfuric acid (H2SO4) to form methanesulfonic (CH4O3S) acid without by-products. The reaction appeared to proceed via a cationic chain mechanism initiated by adding a low concentration of sulfonyl peroxide, propagated by methenium (CH3+) molecules.
Direct functionalization of methane to form value-added products is a challenge due to potential overoxidation in many reaction environments and sulfonation is an attractive approach to achieve the selectivity of interest. In the practical process, Díaz-Urrutia and Ott produced methanesulfonic acid (MSA) using only two main reactants; methane and sulfur trioxide. They achieved 99 percent selectivity and yield of MSA in the work. The scientists based the electrophilic initiator on a sulfonyl peroxide derivative, which they protonated under superacidic conditions to produce a highly electrophilic oxygen atom capable of activating a C-H bond of methane. They proposed mechanistic studies to support the formation of a cation methenium (CH3+)as a key intermediate during the reaction. The proposed method is scalable with reactors connected in a series to prospectively produce up to 20 metric tons of MSA per year.
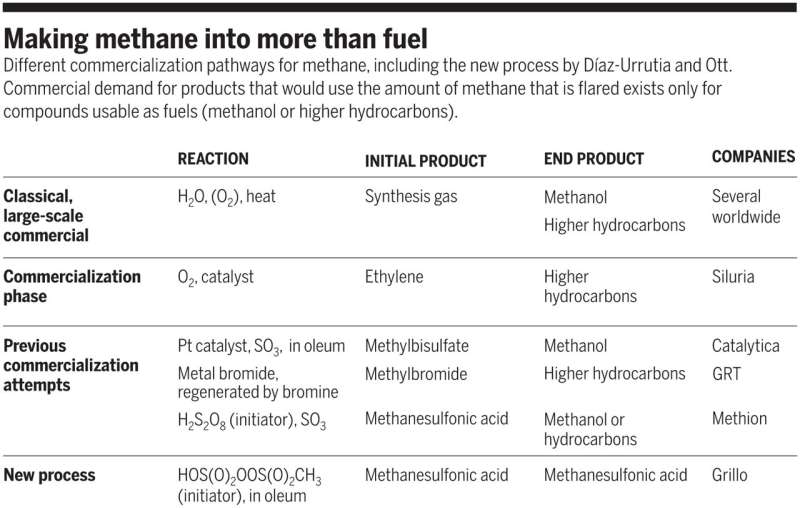
While large-scale fracking techniques and biogas production has provided access to large quantities of inactive methane, the largest chemical transformation of methane remains confined to the highly energy demanding Fischer-Tropsch processes. At present, methane is industrially converted to Syngas, a mixture of carbon monoxide and hydrogen, to form useful products including methanol and Fischer-Tropsch hydrocarbons, which are synthesized in subsequent steps. The production of syngas is severely cost-limiting, however; "MegaMethanol" plants or the Fischer-Tropsch pearl complex in Qatar exceed 10 million metric tons (MT) of the total annual hydrocarbon production. As a result, the direct conversion of methane to valuable products on an economically viable technique are of extreme interest.
Making methane into more than fuel. Different commercialization pathways for methane, including the new process by Díaz-Urrutia and Ott. Commercial demand for products that would use the amount of methane that is flared exists only for compounds usable as fuels (methanol or higher hydrocarbons). Credit: Science, doi: 10.1126/science.aav0177
In this context, the potential to sulfonate methane (CH4) to methanesulfonic acid (CH4O3S, MSA) has achieved substantial attention due to the abundance of both raw materials and the ability for its rapid integration into existing industrial chemical processes. MSA is biodegradable and nonoxidizing with potential applications in metal recycling, energy storage and biodiesel production. Preceding work on methanesulfonation suffered from low yields and conversions, due to free-radical recombination, resulting in undesired side-products such as ethane, rendering the methods unsuitable for large-scale production. Technically, the balance between reactivity and selectivity required by an industrial process can be provided by superacid chemistry. Díaz-Urrutia and Ott reported on the treatment of oleum (20 to 60 percent sulfur trioxide) with CH4 at approximately 500C using less than 1 mol percentage of the electrophilic initiator to form MSA with 99 percent yield and 99 percent selectivity.
CH4(g) + SO3(l) → CH3SO3H(l)
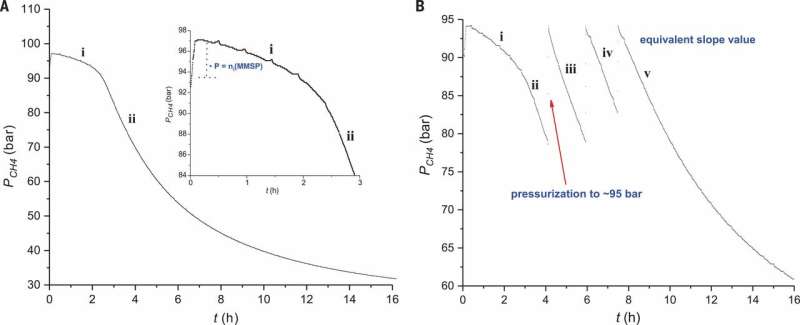
Reaction profile for methane sulfonation. Pressure of CH4 is plotted versus time under (A) standard conditions using 0.9 mol % electrophilic initiator (Figure 2, entry 2) and (B) successive additions of CH4 (Fig 2, entry 3). The inset in (A) shows a zoomed-in view of region i. Credit: Science, doi: 10.1126/science.aav0177.
The scientists first studied the reaction in a batch system to optimize experimental conditions and gain further insight into the reaction mechanism. For the electrophilic initiator, they used monomethylsulfonylperoxide sulfuric acid (MMSP) to improve technical feasibility. For increased productivity, they used a four-liter reactor instead of a 400-mL reactor, owing to larger quantities of CH4 forming in the headspace of the larger reactor. The scientists were thus able to maintain constant amounts of methane throughout the reaction for higher yields of MSA. They used an optimal temperature of 500C to achieve more than 99 percent selectivity towards MSA, whereas previous radical pathways had similar results at higher temperatures (850C) due to thermal decomposition of the sulfonyl peroxide electrophilic initiator. Low-temperature experiments could also offer high conversion and MSA selectivity, but required longer reaction times. Díaz-Urrutia and Ott comparatively provided insights to support a non-radical mechanism in the present work.
When the scientists examined the reaction profile of the experiment, they observed an induction period immediately after addition of the electrophilic reactor, where the amount of MSA (product) was proportional to the initial quantity of MMSP (initiator). At stage two of the reaction profile, they observed the solubility of CH4 decrease with increasing pressure in the reactor. The activation energy of the process was determined to be 111±1 kJ/mol, similar to those previously reported. The described cationic pathway occurred under very specific conditions. The researchers achieved high selectivity through electronic changes in electrophilic substitutions, as opposed to the previously reported free-radical based atom abstraction reactions.
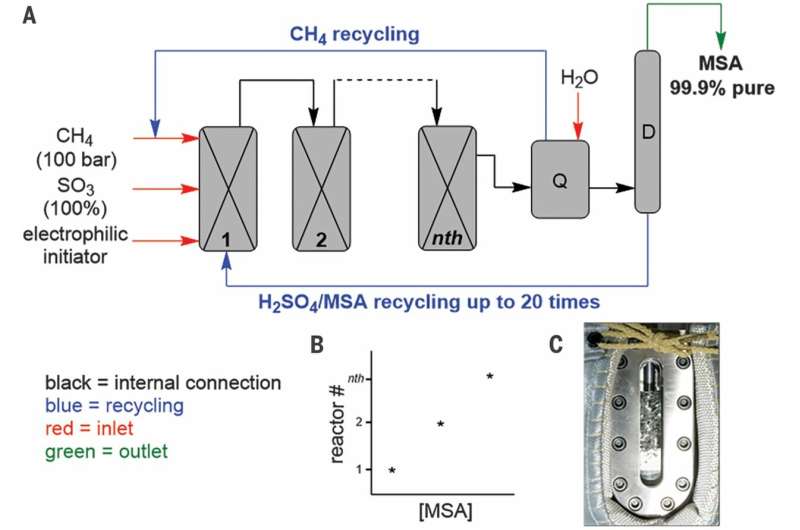
Sulfonation of methane to MSA. (A) Schematic of the Díaz-Urrutia and Ott process. The reaction proceeds as a cascade through reactors connected in series. The pilot plant could produce up to 20 metric tons of MSA per year. The excess SO3 is quenched in reactor Q, the CH4 excess stream and the MSA/H2SO4 sump stream are recycled back to reactor 1, and the MSA-enriched mixture is distilled in column D to obtain pure MSA. (B) The concentration of MSA increases as it passes through the reactors. (C) Oblong quartz window reactor with gas impeller, for improved CH4 mixing. Credit: Science, doi: 10.1126/science.aav0177.
Since the initial results were very promising, the scientists built a pilot plant facility and tested the economic and technical feasibility of industrial scale MSA production. Díaz-Urrutia and Ott constructed the plant with a projected capacity of 20 metric tons/year of MSA production, based on their laboratory-scale batch reactions, and accounted for methane solubility and recycling, as well as for the concentration of sulfur trioxide and methane. This configuration allowed the scientists to constantly increase the concentration of MSA as the reaction mixture passed through the reactors. When they used gas chromatography with flame-ionization detection (GC-FID) to monitor the samples, they did not detect the presence of higher alkanes in the recycled stream of methane or any other radical recombination products, allowing its direct use as feedback stock for the cascade reaction.
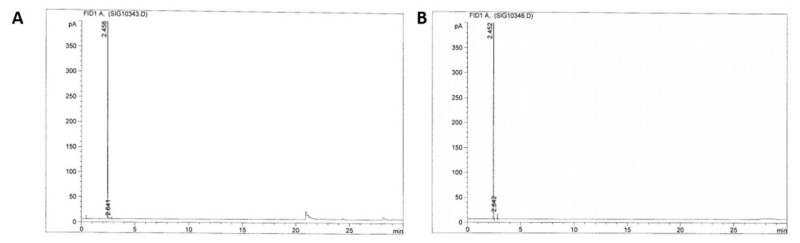
GC-FID Chromatograms. A) GC-FID chromatogram of the gas phase of the reactor (100 bar) before sulfonation of methane has occurred. B) GC-FID chromatogram of the gas phase after (~40 bar) the sulfonation of methane has occurred (16 h, fuming sulfuric acid 36%, 500C). Credit: Science, doi: 10.1126/science.aav0177.
To afford pure MSA, Díaz-Urrutia and Ott completed the process by a final distillation step. They then recycled the remaining mixture of sulfuric acid and MSA to the first reactor for continued regeneration of sulfur trioxide and sulfuric acid (SO3 and H2SO4). Using the four reaction chambers of the setup, the scientists were able to produce 200 kg of pure MSA per week, amounting to two to three metric tons in 80 days. In this way, the demonstrated combination of high selectivity, conversion and atom economy made the process ideal for large-scale valorization of the readily available methane and sulfur trioxide reagents.
If this new process of methanesulfonic acid becomes successful in the market, cheaper reagents will be able to replace the mineral acids presently in use. However, even if the production of MSA were to increase dramatically, the amount of methane consumed in the process would still be dwarfed by the amounts flared. Nevertheless, the work of Díaz-Urrutia and Ott predicts a new synthetic chemical process to synthesize an interesting chemical, allowing the scientists to envision a range of value-added products to be derived from methane or higher alkanes using this route of superacid chemistry in the future.
More information: Christian Díaz-Urrutia et al. Activation of methane to CH3+: A selective industrial route to methanesulfonic acid, Science (2019). DOI: 10.1126/science.aav0177
Ferdi Schüth. Making more from methane, Science (2019). DOI: 10.1126/science.aaw7738
Christopher D. Elvidge et al. The potential role of natural gas flaring in meeting greenhouse gas mitigation targets, Energy Strategy Reviews (2018). DOI: 10.1016/j.esr.2017.12.012
Eric C. D. Tan et al. Reduction of greenhouse gas and criteria pollutant emissions by direct conversion of associated flare gas to synthetic fuels at oil wellheads, International Journal of Energy and Environmental Engineering (2018). DOI: 10.1007/s40095-018-0273-9
Journal information: Science
© 2019 Science X Network