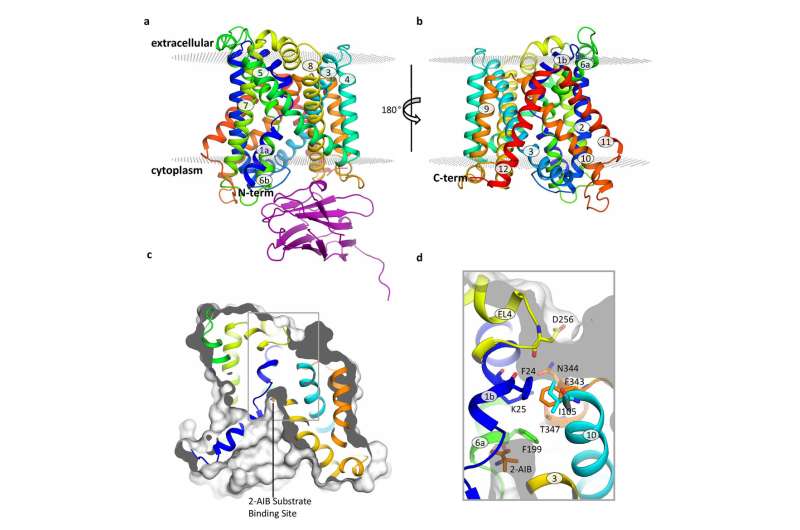
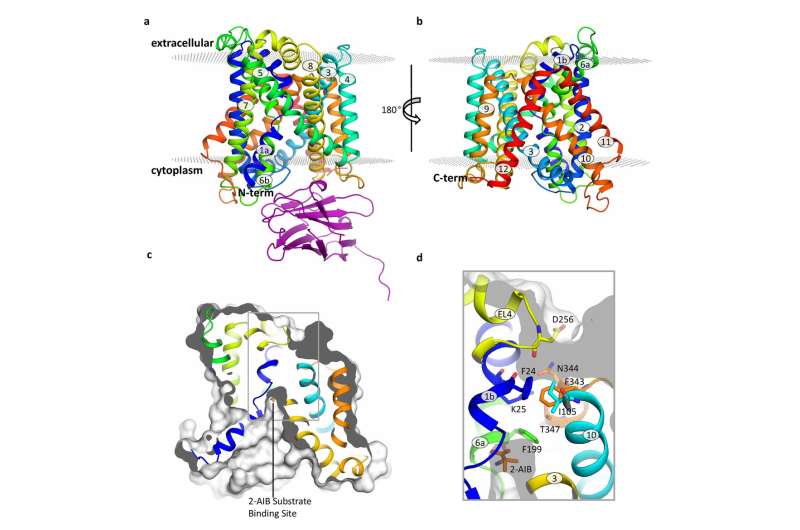
Structure of the BasC transporter. Credit: Manuel Paladín, IRB Barcelona
In humans, there are more than 50 types of amino acid transporters, which are responsible for the cellular uptake of amino acids and for regulating the intra- and extra-cellular balance of these molecules. Mutations in these transporters are associated with a variety of diseases. However, in spite of the importance of these molecules, little is known about how they work. Scientists at the Institute for Research in Biomedicine (IRB Barcelona) have now characterised the structure of a member of the LAT family.
LATs form a family that includes various kinds of amino acid transporters, and are even present in some types of bacteria. Mutated LATs are associated with conditions as diverse as autism, age-related hearing loss, cystinuria and lysinuric protein intolerance.
Published in the journal Nature Communications, this study has focused on BasC, a LAT present in bacteria. "We have used BasC as a model because it shows structural and functional similarity to LATs in humans," explains Manuel Palacín, head of the Amino Acid Transporters and Disease Lab at IRB Barcelona.
By analysing the structure and function of BasC, the first authors of this work, Ekaitz Errasti-Murugarren, Joana Fort and Paola Bartoccioni, postdoctoral fellows at IRB Barcelona, have identified the site at which amino acids bind to LATs and the mechanism underlying the interaction at both sides of the cell membrane.
"Knowing how LATs recognise the amino acids to be transported into the cell allows us to better understand their function and to decipher the molecular defects associated with various conditions. This knowledge will then help us to develop potential treatments," comments Palacín, professor at the Faculty of Biology at the University of Barcelona, and head of unit U731 of the Centro de Investigación Biomédica en Red de Enfermedades Raras (CIBERER).
In this collaboration between IRB Barcelona, CIBERER, IBMB-CSIC and the Barcelona Supercomputing Center, the researchers have characterised the structure of BasC by means of X-ray crystallography and using an antibody against this transporter. "The BasC antibodies that we developed provide an excellent tool through which to study the function of this transporter," adds Palacín.
Structure of the BasC transporter. Credit: Manuel Palacín, IRB Barcelona
More information: Ekaitz Errasti-Murugarren et al, L amino acid transporter structure and molecular bases for the asymmetry of substrate interaction, Nature Communications (2019). DOI: 10.1038/s41467-019-09837-z
Journal information: Nature Communications