Tumor-promoting enzymes USP25 and USP28: Substantial differences identified
Researchers from the Rudolf Virchow Center of the University of Würzburg (JMU) have solved the structures of the cancer-promoting enzymes USP25 and USP28, and identified significant differences in their activities. Both enzymes promote the growth of tumors. The results were published in the journal Molecular Cell and could benefit towards the development of new, low-side-effects anticancer drugs.
The permanent interplay of protein production and degradation is a major driver of cellular metabolism. A key mechanism of this regulation is the labeling of a proteins with ubiquitin, a small polypeptide that plays important roles in many pathways, but also acts as a signal for protein degradation.
Ubiquitination can be reversed by a group of enzymes, the so-called deubiquitinases, which are able to rescue the proteins. In cancer cells these opposing processes are assumed to be out of balance. Deubiquitinases have therefore emerged in recent years as promising drug targets for cancer therapy.
Structures of the catalytic domains
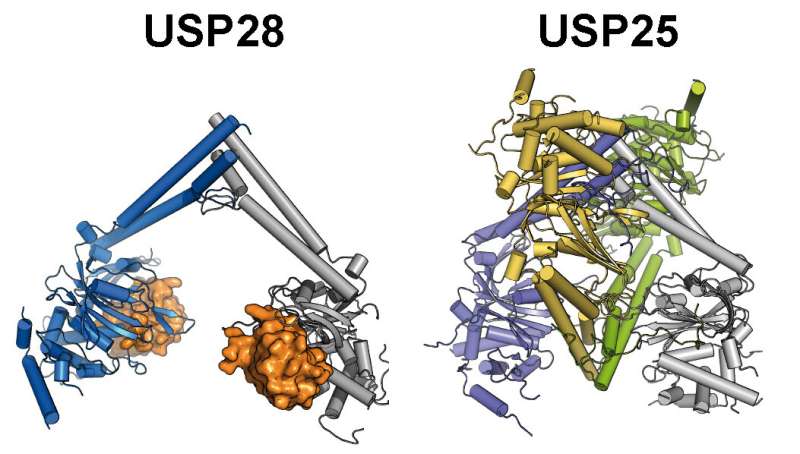
The study focused specifically on the deubiquitinases USP25 and USP28. Both enzymes are important for the progression of various types of tumors. The research of the two structural biologists Dr. Florian Sauer and Theresa Klemm from Prof. Caroline Kisker's group at the Rudolf Virchow Center provides important insights into the structure and function of the two deubiquitinases. Using X-ray crystallography, the group was able to solve the three-dimensional structure of the central catalytic domains of both evolutionarily closely related enzymes and they uncovered similarities as well as crucial differences.
"It was surprising to discover that USP25, despite its structural similarity to USP28, is inactive in the isolated form (in vitro) as well as in cells (in vivo), and that there is a correlation between the oligomeric state and its activity," says Sauer. The researchers' analysis showed that the inactive USP25 forms a tetramer, a molecular complex of four-subunits. In contrast, USP28 exists as a constitutively active dimer, a molecular complex of two identical subunits. "By inserting alterations, found in cancer cells, we were able to show that the USP25 tetramer dissociates into two USP28-like dimers, thereby abolishing the auto-inhibition of the enzyme," reports Klemm.
A new guide for cancer drugs
This knowledge provides the molecular basis for the development of new and highly specific anti-cancer drugs, with a low risk of side-effects. "On the one hand, new approaches have been identified that could, for example, be used to develop tailor-made drugs targeting specifically USP28, thereby preventing inhibition of USP25, which is important for the immune response," says Sauer. "On the other hand, specific targeting of the already per se inhibited USP25 seems feasible by preventing its activation," adds Klemm.
More information: Florian Sauer et al, Differential Oligomerization of the Deubiquitinases USP25 and USP28 Regulates Their Activities, Molecular Cell (2019). DOI: 10.1016/j.molcel.2019.02.029
Journal information: Molecular Cell
Provided by University of Würzburg