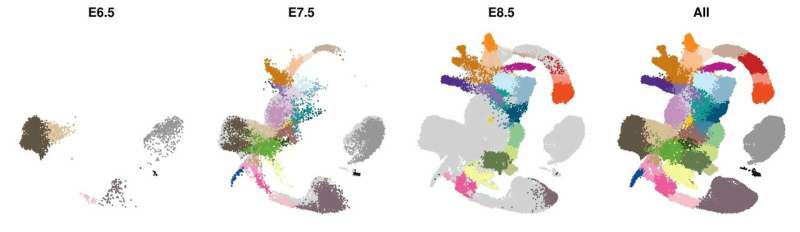
Each dot represents a single cell in the developing embryo, with the number of specialized cells increasing across the 48hrs from embryonic day 6.5 to embryonic day 8.5. The dots are colored based on which major cell type they represent, such as heart, lungs, brain and gut. The 116,000 dots are arranged so that cells with similar genetic activity are close to each other. This new Molecular Map is freely available to researchers to give them access to the complete genetic programs that drive the development of all major organ systems, and opens up new avenues for regenerative medicine and drug development. Credit: Wellcome-MRC Cambridge Stem Cell Institute
A team of biologists, physicists and mathematical modellers in Cambridge have studied the genetic activity of over 100,000 embryonic cells to establish the molecular blueprint of mouse early embryo development. This new research provides fundamentally important information on how mammalian embryos develop during gastrulation, a key stage of development, and paves the way for new understanding of the earliest stages of life.
The eminent biologist Lewis Wolpert famously stated that "It's not birth, marriage or death, but gastrulation that is truly the most important time in your life". Gastrulation represents the process in animal (and human) development whereby precursor cells in the embryo become genetically programmed to generate all of the different organs in the body, including the brain, heart, lungs, gut and muscles. Gastrulation is a critically important step in embryo development, however, detailed understanding of this process at the molecular level has, until now, been limited.
In a study published in Nature, Cambridge scientists have generated the first comprehensive molecular map of gastrulation in mammals. Measuring the genetic activity in 116,312 single cells within the mouse embryo between 6.5 to 8.5 days after fertilisation, the researchers have established the molecular blueprint for mammalian embryonic development.
The researchers used cutting-edge single-cell technology to measure which genes are activated in the mouse embryo across a number of sequential time-points. Beginning with only a small number of distinct cell-types detected at the start of gastrulation, the cells branch out into over 30 different cell types with different genetic profiles, all within a time span of only 48 hours.
Computational analyses enabled the scientists to generate "interactive maps" where each cell is represented by a dot, and cells with similar molecular profiles are positioned close to each other. These new maps, which are freely available online for other researchers to use, illustrate the trajectories of cellular development from one cell type to the next, and show the precise genetic processes that enable all of the cells and organs of the body to develop from their early embryonic origins.
"Our new map provides a molecular blueprint to outline how embryonic development proceeds under normal conditions. It allows us to see which different sets of genes are activated when unspecified embryonic cells proliferate and diversify into all the specific cell types across the body" said Professor Bertie Göttgens, group leader at the Wellcome-MRC Cambridge Stem Cell Institute. "The map is also an invaluable reference point to understand how genetic mutations can disrupt embryo growth and cause developmental disorders and diseases."
The researchers tested the new molecular map by investigating Tal1, a gene that is essential for normal blood development, yet if activated in the wrong cells, can cause leukaemia. By comparing the reference atlas to over 10,000 Tal1 mutant cells, the researchers were able to decipher the consequences of the Tal1 genetic mutation.
"To investigate the role of Tal1 in blood development, we mixed embryonic stem cells carrying the mutated Tal1 gene with very early normal embryos to make chimaeras," explained Professor Jenny Nichols, group leader at the Wellcome-MRC Cambridge Stem Cell Institute. "Because these chimaeras could still make blood from their normal cells, we were able to study the mutant cells in their proper biological context."
Dr. John Marioni, group leader at EMBL's European Bioinformatics Institute (EMBL-EBI) and the Cancer Research UK Cambridge Institute said: "By comparing our experimental data with the data collated within the molecular map, we were able to decipher precisely what was going on within the cells with mutant Tal1 genes. We could see that the mutant cells weren't simply getting stuck, or deciding to become a different cell-type, but instead the cells started expressing a wide range of different genes, as if they were confused about what cell type they should mature into."
Investigating the genetic basis of blood development is just one way the new molecular map could be used to understand normal and disease processes. The extensive nature of the map, which contains molecular information on all of the developing cell types in the embryo, will allow other researchers to gain a deeper understanding of how a whole range of organs develop. This in turn will enable scientists to develop new protocols for the production of authentic cell types for drug screening, as well as therapies aiming to regenerate diseased or ageing organs.
Dr. Sheny Chen from Wellcome's Cellular and Developmental Sciences team said: "By piecing together this 'molecular map' of how cells develop in a mouse embryo, the researchers have created an important and useful resource for the research community. Science is a collaborative effort and this team of biologists, physicists and mathematicians have been able to gain new understanding of gastrulation, a pivotal process in development which generates the three cell layers that give rise to all the organs of the body."
More information: A single-cell molecular map of mouse gastrulation and early organogenesis, Nature (2019). DOI: 10.1038/s41586-019-0933-9 , www.nature.com/articles/s41586-019-0933-9
Journal information: Nature
Provided by University of Cambridge