A RUDN Chemist Developed a New Catalyst for Oxidation and Amidation Credit: Allen Dressen
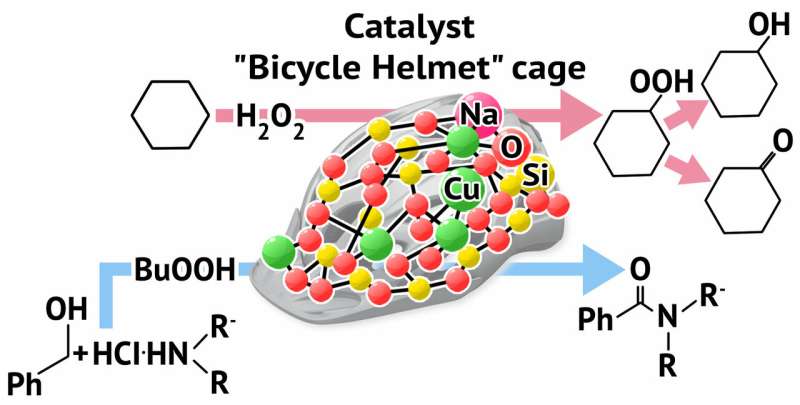
A RUDN chemist has obtained a compound with a new structural type containing atoms of metals (copper and sodium) in a carcass structure and that is shaped like a bicycle helmet. The compound shows catalytic activity in two important organic synthesis reactions. The development may be used to create new catalysts for chemical industry. The work was published in the Dalton Transactions journal.
One promising area of modern chemistry is the formation of complex molecular systems containing a set of metals within one structure. To effectively obtain such an object, researchers use several types of ligands (substances surrounding metal atoms). For example, scientists have already obtained polymetallic complexes in which ligands coordinate metals with different centers (nitrogen/sulphur, phosphorus/nitrogen, etc). Using this approach, researchers can develop objects with different properties.
A RUDN chemist and Russian and French colleagues have synthesized a new carcass product based on copper and sodium atoms. The compound also contains two types of framework for the metals—an oxygen one (siloxane ligand) and a nitrogen one (bipyridine). To develop a silicon-organic ligand, a reaction of interaction between a simple silicon-organic compound called phenyltrimethoxysilane PhSi(OCH3)3 and sodium hydroxide was carried out in a solution. Then, copper dichloride and the second ligand (the organic compound bipyridine containing two atoms of nitrogen that quickly form complexes with metals) were added to the solution.
The target compound was obtained after the correct ratio between the reaction agents and the correct environment for the reaction/crystallization were found. X-ray structural studies showed an unusual molecular geometry of the product, a rarely occurring ratio between metals in the carcass (5 atoms of copper and 1 atoms of sodium), and unusual intermolecular reactions between the neighbouring carcasses in the crystal. They are united in the so-called supramolecular system due to the interconnection of aromatic rings of the nitrogen ligand.
"We've obtained a new type of carcass metal complex with nitrogen and oxygen centers in the framework of copper and sodium ions. This type of bonding forms unusual molecular architecture that resembles a bicycle helmet. We believe that our approach—the construction of metal complexes using different types of ligands—may be developed and improved, and we expect to obtain previously unknown chemical objects. Moreover, such mixed framework of metal atoms may play a positive role in catalysis by supporting their activity," says Alexey Bilyachenko, a co-author of the work, a Candidate of Chemistry, and a RUDN staff member.
During the second stage of the study the scientists measured the catalytic activity of the new compound in oxidation and amidation reactions. The "bicycle helmet" proved to be effective in the catalysis of reaction of cyclohexane and n-heptane with peroxides, which is a promising result for obtaining reactive oxidation products. Such products may be further used to obtain various materials, including polymers. The 'helmet' also showed high activity in reactions between benzyl alcohol and amines. The reaction is of great importance as a source of amides (components of medicinal drugs). The 'helmet' remained active in catalysis even when used in small quantities (0.4 mol% of copper).
More information: Alena N. Kulakova et al. A new "bicycle helmet"-like copper(ii),sodiumphenylsilsesquioxane. Synthesis, structure and catalytic activity, Dalton Transactions (2018). DOI: 10.1039/C8DT03209B
Journal information: Dalton Transactions
Provided by RUDN University