Silicones obtained at low temperatures with the help of air

Russian scientists have developed a new method for synthesizing para-carboxyplenylsiloxanes, a unique class of organosilicon compounds. The resulting compounds are promising for creating self-healing, electrically conductive, heat- and frost-resistant silicones.
Organosilicon compounds, especially materials based on silicones, are among the most in-demand products. The ability to withstand incredible thermal and mechanical stress makes it possible to use silicones for sealing and protecting many items in aircraft and rocket construction. The strength and durability of silicones lends them to applications in medicine, food industry, and in many other fields of human life.
Though many silicone materials have already been created and their fields of application have been found, scientists believe that their usability potential has not been fully realized. This is due to one of the central problems in the modern chemistry of silicones, namely, the synthesis of organosilicon products with a "polar" (-C(O)OH, -OH, -NH2, etc.) functional group in an organic substituent. Such a moiety allows the easy introduction of other substituents, and the ability to tune the compound to repel water or to form stable aqueous emulsions, and to impart other "super-capabilities" to a material. This opens quite unique prospects for subsequent modification of these compounds in order to synthesize new copolymers, self-healing and conductive materials, and compounds for the storage and delivery of drugs and fuels. Just a small modification of a compound would also allow one to solve the problem of low mechanical strength and incompatibility of silicones with polymers, such as polyesters and others.
With rare exceptions, the classical methods for synthesizing silicones (first monomers, then polymers) cannot realize functional organosilicon substrates. As a rule, these methods are either applicable to a narrow range of substrates or are time-consuming, expensive and involve multiple stages.
In recent years, there have been an increasing number of publications on the oxidation and functionalization of organic compounds involving molecular oxygen, i.e., a "green," simple and available oxidant. A number of industrially important processes already rely on this approach. However, despite all the advantages, these processes generally feature low selectivity and require drastic conditions (elevated temperature, high pressure, etc.).
A team of scientists from A.N. Nesmeyanov Institute of Organoelement Compounds of the Russian Academy of Sciences (INEOS RAS), in collaboration with colleagues from the Russian Federation, used a combination of metallic and organic catalysts to solve these problems. The reaction conditions were softened and high process selectivity was achieved. The reaction occurred with involvement of molecular oxygen in liquid phase and at temperatures slightly above the room temperature, whereas many industrial processes are performed in gas phase under drastic conditions. The method can be scaled to gram amounts in order to produce a required compound.
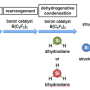
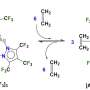
"Thus, we suggested a highly efficient method based on aerobic metal- and organo-catalyzed oxidation of starting para-tolylsiloxanes to para-carboxyphenylsiloxanes. This approach is based on 'green,' commercially available, simple and inexpensive reagents, and employs mild reaction conditions," says Dr. Ashot Arzumanyan, the leader and one of the contributors of this study, senior scientist of the K.A. Andrianov Laboratory.
Furthermore, it has been shown that the suggested method is applicable to the oxidation of organic derivatives (alkylarenes) to the corresponding acids and ketones, as well as hydridosilanes to silanols (and/or siloxanols). The scientists also studied whether materials can be obtained on the basis of para-carboxyphenylsiloxanes, including an analogue of PET, which is used in beverage bottles, fibers for clothes and for technical applications. "The compounds that we obtained open prospects for the creation of self-healing, electrically conductive, heat- and frost-resistant and mechanically strong silicones. They can also serve as a basis for developing new hybrid materials that may find use in catalysis, drug delivery, fuel storage, and in other fields of science, technology and medicine," Ashot notes.
More information: Irina K. Goncharova et al, Aerobic Co- / N-hydroxysuccinimide- catalyzed oxidation of p-tolylsiloxanes to p-carboxyphenylsiloxanes: synthesis of functionalized siloxanes as promising building blocks for siloxane-based materials, Journal of the American Chemical Society (2019). DOI: 10.1021/jacs.8b12600
Journal information: Journal of the American Chemical Society
Provided by AKSON Russian Science Communication Association