Shiftless: Novel host antiviral factor that inhibits programmed -1 ribosomal frameshifting
The genome sizes of viruses are usually relatively small. To increase information content of the genome, many viruses employ a translation recoding mechanism dubbed programmed ribosomal frameshifting.
Translating ribosomes pause at a -1PRF signal. While most ribosomes move on in the original reading frame, a small proportion slip back one nucleotide to translate in a new frame, resulting in two protein products differing at the C-termini. HIV-1 uses programmed -1 ribosomal frameshifting (-1PRF) to produce Gag and Gag-Pol, which are both required for viral replication.
The -1PRF mechanism exists in all domains of life. In eukaryotes, -1PRF may also result in a premature stop codon, which could lead to the degradation of mRNA. The -1PRF mechanism plays an important role in the post-transcriptional regulation of gene expression. However, how -1PRF is regulated by host factors is largely unknown. In a study published in Cell, GAO Guangxia's group at the Institute of Biophysics of the Chinese Academy of Sciences reported a novel host antiviral factor named Shiftless that inhibits -1PRF.
GAO's lab has been focusing on the molecular mechanism underlying virus-host interactions. To identify host factors that inhibit -1PRF, they demonstrated that type I interferon can inhibit the expression of Gag-Pol, the -1PRF product of HIV-1. By screening interferon-stimulated genes (ISG) for their capacity to inhibit Gag-Pol expression, they identified Shiftless (originally named C19orf66).
Shiftless displayed considerable inhibitory activity against all the tested -1PRF from both viruses and cellular genes, indicating that it is a broad-spectrum -1PRF inhibitor.
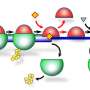
To explore the mechanism of Shiftless, researchers analyzed the interactions of Shiftless with the -1PRF RNA and translating ribosomes, two key players in the process of -1PRF. Shiftless interacted with both. Based on this result, they reasoned that Shiftless binding to the translating ribosomes and RNA simultaneously might render the ribosome stuck in a non-productive state, stalling on the RNA. The stalled ribosome should be rescued by the quality control mechanism, leading to premature translation termination.
Using a sensitive reporter system, they detected the premature translation termination product, proving their hypothesis. They demonstrated that the premature translation termination was executed by the host translation release factors eRF1 and eRF3.
Moreover, researchers proposed a working model for Shiftless to inhibit -1PRF. Shiftless interacts with the -1PRF signal RNA and the translating ribosome, and thereby causes ribosome stalling at the -1PRF site. Furthermore, Shiftless recruits the translation release factors eRF1-eRF3 to rescue the stalled ribosome, resulting in the production of premature translation termination (PMT) product.
Since -1PRF is a widely used mechanism, these results have far reaching implications that may impact many different fields.
More information: Xinlu Wang et al, Regulation of HIV-1 Gag-Pol Expression by Shiftless, an Inhibitor of Programmed -1 Ribosomal Frameshifting, Cell (2019). DOI: 10.1016/j.cell.2018.12.030
Journal information: Cell
Provided by Chinese Academy of Sciences