January 25, 2019 report
Previously unknown crystalline phase of semi-aqueous calcium carbonate discovered
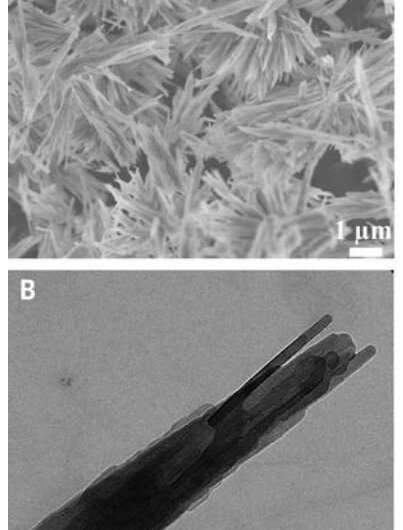
A team of researchers from Germany, Israel and the U.S. has discovered a new crystalline phase of semi-aqueous calcium carbonate. In their paper published in the journal Science, the group describes working with magnesium ions in crystalline pathways of calcium carbonate when they discovered the crystalline phase.
Calcium carbonate is one of the most prevalent materials found on Earth—it forms from the skeletal remains of marine creatures over long periods of time. The minerals that result are used in a wide variety of industrial processes and products. Only three kinds of CaCO3 in its pure state are known— valerite, aragonite and calcite, as well as two hydrated crystal phases—monohydrocalcite and ikaite. The new crystalline phase adds a third— hemihydrate CaCO3·½H2O.
The researchers note that calcium carbonate is among the most important parts of the geochemical cycle of carbon—it makes up approximately 4 percent of the Earth's crust. Because of its abundance, scientists in various fields are curious about its nature. Prior studies of its makeup have illuminated climate history and the acidity of the oceans.
The researchers report that the new phase forms from ACC when bathed in a solution of magnesium ions. They report also that they were able to synthesize samples of the material using magnesium chloride, sodium carbonate and calcium chloride. More specifically, team used a three-stage process. In the first, an amorphous monohydrate of calcium carbonate was formed, comprising approximately 6.5 percent of the MG's molar content. In the second stage, as the activity of calcium ions fell sharply (after approximately 20 minutes), the magnesium content decreased to 1.5 times that of the molar percentage, and the pH increased. The third stage lasted approximately 11 hours, during which time the semi-aquatic crystallological draant passed into monohydrate, along with a decrease in Ph and CA2+ activity. The researchers also offered a comprehensive description of the structures that formed, including formation pathways.
The researchers suggest that naturally occurring calcium carbonate with the new phase (if found) could prove useful for better understanding its nature and possible applications for its use.
More information: Zhaoyong Zou et al. A hydrated crystalline calcium carbonate phase: Calcium carbonate hemihydrate, Science (2019). DOI: 10.1126/science.aav0210
Journal information: Science
© 2019 Science X Network