Machine learning and quantum mechanics team up to understand water at the atomic level
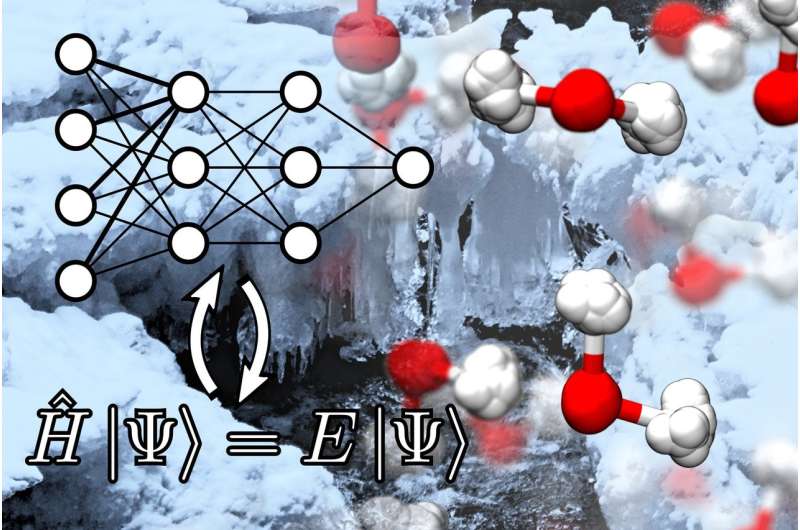
Why is water densest at around 4 degrees Celsius? Why does ice float? Why does heavy water have a different melting point compared to normal water? Why do snowflakes have a six-fold symmetry? A collaborative study, led by researchers in EPFL and just published in the Proceedings of the National Academy of Sciences, provides physical insights into these questions by marrying data-driven machine learning techniques and quantum mechanics.
The building blocks of most observable matter are electrons and nuclei. Following the laws of quantum mechanics, their behavior can be described in terms of their wave function, sort of a diffuse cloud that is related to the probability of observing them in a given point and time. By solving the Schrodinger equation, it is possible to make models and predictions of any material, including water. But there is a catch. As the number of electrons and nuclei increases, the complexity involved soon becomes intractable, even with the fastest supercomputers, despite a century of celebrated progress in optimizing such calculations. In fact, quantum mechanical calculations are still unaffordable for systems with more than a few hundred atoms, or for a time period longer than a nanosecond.
To overcome these harsh limitations, the researchers exploited an artificial neural network (ANN) to learn the atomic interactions from quantum mechanics. The architecture of ANNs can be represented as several layers of interconnected nodes that mimic the structure of the neurons in a human brain. The ANN first learns quantum mechanical interactions between atoms, and then makes speedy predictions about the energy and forces for a system of atoms, bypassing the need to perform expensive quantum mechanical calculations.
So far, it all rather sounds like just another success story of machine learning. However, there are subtleties. The ANN has a residual error compared to the actual quantum mechanical calculations: Most of the time, it introduces a small amount of noise, and sometimes it makes a wild guess—this happens when an input is very different from anything it has already learned.
How to avoid the pitfalls of the ANN: Instead of employing ANN on its own to make predictions about a system of atoms, the researchers used it as a surrogate model. In essence, computing properties of materials at a finite temperature usually involves many computation steps, and the laborious and repetitive parts can be delegated to the cheap surrogate model. Finally, the difference between the surrogate and the ground truth, which is the difference between the ANN and quantum mechanics, can be accounted for and subtracted from the final predictions.
With these techniques, the researchers were able to to reproduce several thermodynamic properties of water from quantum mechanics, including the density of ice and water, the difference in melting temperature for normal and heavy water, and the stability of different forms of ice. Moreover, the study reveals several physical insights on what give ice and water systems their peculiar properties. One of the most notable findings is the that nuclear quantum fluctuations, which involve the tendency for light elements such as hydrogen to behave more like a diffuse cloud rather than a localized particle, promote hexagonal packing of molecules inside ice, which ultimately leads to the six-fold symmetry of snowflakes.
More information: Bingqing Cheng et al, Ab initio thermodynamics of liquid and solid water, Proceedings of the National Academy of Sciences (2019). DOI: 10.1073/pnas.1815117116
Hirofumi Sato et al. Ab initio study of water. II. Liquid structure, electronic and thermodynamic properties over a wide range of temperature and density, The Journal of Chemical Physics (2002). DOI: 10.1063/1.480195
Journal information: Proceedings of the National Academy of Sciences , Journal of Chemical Physics
Provided by University of Vienna