New insight into unique sugar transport in plants
Sugar transport through sugar transport proteins (STP) is unique to plants, and is important for the proper development of plant organs such as pollen. STPs are also used to concentrate sugars in specific tissues like fruit, and they play an important role in the plant defence against fungal attacks from things like rust and mildew.
Sugar is generated in plant leaves by photosynthesis, and is transported as the disaccharide sucrose to other parts of the plant through the sieve tissue. In sink tissues such as roots, pollen and fruits, the plant can absorb the sugar either as sucrose or, after cleavage, as the monosaccharides glucose and fructose.
Uptake of glucose and other monosaccharides is driven by STPs that move sugar through the otherwise impermeable cell membrane using an acid gradient. These proteins have some specific properties compared to similar proteins from animals or bacteria. They have an extremely high affinity for sugar; in fact, they bind 1000 times more strongly to sugars than similar proteins in humans. At the same time, they maintain a very high level of activity over a broad pH spectrum compared to other acid-driven sugar transporters.
First glance at a unique transport mechanism
A small group of researchers from the Department of Molecular Biology and Genetics at Aarhus University in Denmark has solved the structure of a STP sugar transport protein for the first time and provided explanations for the particular transport characteristics of STPs.
STPs are proteins located in the cell membrane that are very difficult to work with. Therefore, it often takes many years to obtain new results, which was the case with this study. The researchers had to change strategy and apply new methods several times.
"It has been an extremely challenging process. Along the way, we have had to let go of very promising results and start all over with new methods because the quality of the data from the traditional structural methods was simply not good enough," says Postdoc Peter Aasted Paulsen, who, as first author, describes the results in the prestigious journal Nature Communications. "It was frustrating to let go of something that was almost good enough to start all over from a new angle, but it was certainly necessary. The many small 'a-ha' moments we gained through our many attempts were what ultimately solved the problems of getting data of sufficiently high quality. It was fantastic when it finally worked."
A new domain surprises
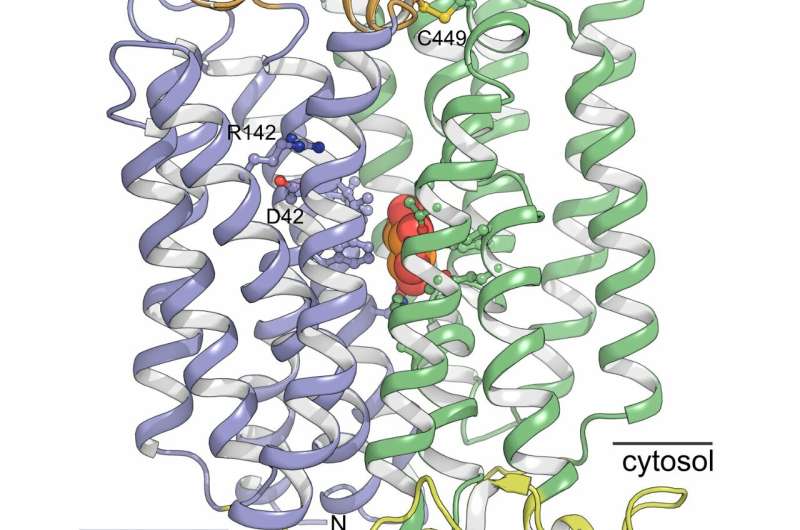
With the new structure, the researchers show that the overall form of the STPs resemble human sugar transporters. But the structure also holds surprises. Peter Aasted Paulsen highlights a new domain that has not been described before. "Over the binding pocket where sugar is located, the STPs have a novel small domain that resembles a lid that is held in place with an unusual bond, a so-called disulfide bridge. It was a completely unexpected observation that immediately sparked the imagination."
To investigate the function of the domain, the researchers made a version of the protein in which this bond was removed. With this change, the protein loses its ability to transport sugar efficiently at certain pH values. If you compare these results with an analysis of the structure, it can be seen that the lid is held in place by means of the bond, thereby creating a favourable environment for acid binding to a specific acid binding pocket. This binding causes a portion of the protein to be pushed toward the sugar molecule, thereby creating the very high affinity for sugar.
"Structural biology is special when it comes to explaining the very detailed mechanisms at play in proteins," says Assistant Professor Bjørn Panyella Pedersen, head of the research group. "It has been extremely satisfying to experience how structure and biochemistry have been combined here to explain something completely fundamental about sugar transport in plants we did not know before."
The results might make it easier to develop resistant crops
"From our slightly nerdy point of view, it has been extremely exciting to be able to answer these very fundamental questions," says Bjørn Panyella Pedersen. "We started working with the human sugar transporters, but since many of the big issues in this field have been answered over the past few years, we decided to turn our focus on sugar uptake in other organisms."
"STPs have very distinct characteristics, and it is very exciting to be able to improve our understanding of how they work," continues Bjørn Panyella Pedersen, "It is especially interesting that the results we now describe are related to how many of the plants' organs develop correctly, and at the same time have proved to be an important contribution to plants' response to fungal attacks. Some species of wheat are fungal resistant, and our results help to explain why."
More information: Peter Aasted Paulsen et al, Crystal structure of the plant symporter STP10 illuminates sugar uptake mechanism in monosaccharide transporter superfamily, Nature Communications (2019). DOI: 10.1038/s41467-018-08176-9
Journal information: Nature Communications
Provided by Aarhus University