December 13, 2018 feature
Enhanced osteogenic activity of pre-osteoblasts on surface-modified 3-D printed scaffolds

Materials such as poly(ε-caprolactone) are used as scaffolds in bone tissue engineering, but their inherent hydrophobicity and surface smoothness can impair cell attachment, proliferation and differentiation in the lab, or after implantation in vivo. Surface modifications including chemical alterations or the immobilization of biologically active molecules on materials can overcome the intrinsic hydrophobicity of poly(ε-caprolactone) (PCL). In a recent study, bioengineers Yasaman Zamani and her colleagues investigated a chemically modified, 3-D printed PCL material surface immobilized with RGD peptide (R: arginine, G: glycine, D: aspartic acid). The results of the study are published on Biomedical Materials, IOP Publishing.
Large bone defects caused by trauma or tumor resection often cannot heal via the natural process of bone regeneration. The existing gold standard for clinical treatment of such defects is autologous bone transplantation; where bone tissue harvested from the same patient at a different surgical site is implanted at the site of injury or defect. The autograft technique is disadvantaged due to limited supply, the need for multiple surgeries, patient's age-related immunocompromise and extended healing time. As a result, bone tissue engineering (BTE) is rapidly becoming a promising alternative that eliminates the need for additional surgeries. By design, BTE creates a scaffold to temporarily substitute the extracellular matrix surrounding the site of defect to assist tissue regeneration and bone repair for a specific timeline. First-generation techniques of BTE cannot control the porosity, microarchitecture and geometry of scaffolds. Three-dimensional (3-D) printing is commonly used at present to engineer scaffolds for tissue engineering with controlled shape and architecture.
The most widely used polymer for 3-D printing bone scaffolds is PCL, due to its low melting and glass transition temperature for easy processing. The polymers have excellent mechanical character suited for bone replacement scaffolds and are approved by the US Food and Drug Administration. However, for cell-seeding applications in BTE, 3-D printed PCL polymers require surface modification as the inherent hydrophobicity and lack of surface-bound biological recognition sites limit surface biocompatibility. A range of existing BTE surface modification techniques are therefore implemented as physical, chemical and biological methods. For instance, hydrolysis of PCL by sodium hydroxide (NaOH) is a chemical technique that can increase hydrophilicity (water-loving nature) of PCL by creating surface carboxyl and hydroxyl groups for enhanced cell attachment.

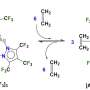
Immobilizing RGD peptide on PCL surfaces could also assist cells to attach and grow on modified surfaces. In this instance, cell-material attachment was attributed to integrins; a group of cell surface proteins that mediate cell bonding to specific adhesion molecules and thereby recognized the RGD sequence on a substrate surface. While the effects of surface modification on biomaterial properties and cellular responses were extensively studied, the results are not applicable for all types of scaffolds. Most importantly, experiments still remain to be conducted to understand which of these surface modifications is more effective for pre-osteoblast cell proliferation and osteogenic activity on a 3-D printed scaffold. Zamani et al. therefore investigated 3-D printed PCL scaffold surfaces modified by alkali treatment with NaOH or by RGD immobilization to understand cellular response on the material construct.
In the study, the researchers conducted biofunctionalization experiments with murine calvariae pre-osteoblasts (MC3T3-E1) to assess the osteogenic response on modified 3-D surfaces for BTE. Chemical surface modification was accomplished using NaOH treatment for 24 hours or 72 hours (3M concentration), which changed the surface topography from a smooth surface to a honeycomb-like structure. For RGD-immobilization, surfaces were incubated with 600 µl of RGD (0.125 mg/ml). In brief, during a timeframe of 1-14 days of cell culture, increased collagenous matrix deposition was observed on the NaOH-treated and RGD immobilized scaffolds compared to the non-modified controls. The chemically modified surfaces showed increased alkaline phosphatase activity, crucial for bone development. The researchers noted that surfaces treated with NaOH for an optimal 24 hours enhanced mineralization compared to the non-modified controls.
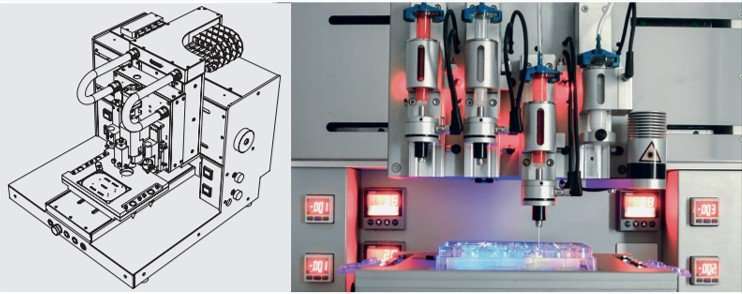
The researchers used a 3-D DiscoveryTM bioprinter to print the scaffolds. The medical grade PCL material was melted in the heating tank and extended through a pre-heated needle, the strands of PCL were plotted layer-by-layer to create 36 cubic scaffolds. The different PCL scaffolds with and without surface modifications were characterized using scanning electron microscopy (SEM). Cell culture was conducted with MC3T3-E1 pre-osteoblasts on the different materials of interest to observe and quantify parameters of cell proliferation, differentiation, collagen-matrix deposition, alkaline phosphatase activity and calcium deposition from 1-14 days. Both 24-hour and 72-hour NaOH treated surfaces showed a honeycomb-like surface topography, but RGD immobilization did not similarly change the surface topography. The cultured pre-osteoblasts were slightly spherical on the unmodified PCL scaffolds indicating surface hydrophobicity, in comparison cells were well spread on the 24-hour NaOH treated and RGD-immobilized scaffolds due to surface hydrophilicity and cell-surface recognition.
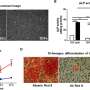
Collagen deposition on the modified/unmodified surfaces cultured with cells were observed with picrosirius red staining using light microscopy at day 14. The quantified intensity of red was greater for 24-hour NaOH treated and RGD-immobilized scaffolds compared to the controls. Additionally, compared to the NaOH scaffolds the RGD-modified surfaces showed significantly higher collagen deposition. Calcium deposition was observed with alizarin red stained scaffold constructs using optical images. More red stain was observed on NaOH treated scaffolds to indicate comparatively more calcium deposition. Similarly, ALP activity was comparatively highest on the 24-hour NaOH treated scaffolds. Interestingly, NaOH treatment for 72 hours did not increase ALP activity compared to the unmodified controls.
Based on the initial results, the parameters of surface modification were refined in the study to include optimal RGD immobilization (0.011 µg/mL scaffold) and 24-hour NaOH treatment to chemically engineer the 3-D printed PCL scaffolds. The study collectively showed improved osteogenic differentiation on 24-hour NaOH-treated scaffolds compared to RGD-immobilized scaffolds in vitro. The results suggested that chemical treatment of 3-D PCL scaffolds using 3M NaOH may be more promising for in vivo bone regeneration studies compared to RGD-immobilization, therefore. Surface modification due to the introduction of functional hydroxyl and carboxyl groups via NaOH treatment increased hydrophilicity and biocompatibility. On the other hand, immobilization of RGD on PCL facilitated cell attachment and proliferation due to sites of cell recognition indicating that both conditions were favorable initially for pre-osteoblast attachment and proliferation in vitro.
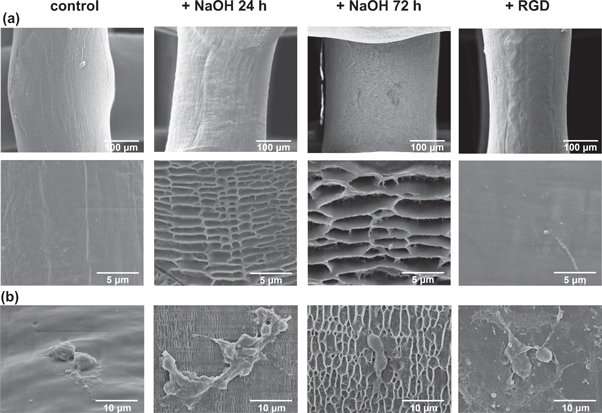
Longer immersion during NaOH treatment (72 hours) was not favorable as increased surface degradation led to higher microscale roughness preventing adequate cell-cell and/or cell-matrix interactions. The results indicated the timescale to attain optimal surface topology (surface roughness and stiffness in this instance) to direct osteogenic differentiation was 24 hours of NaOH-treatment. Cytocompatibility was reiterated with ALP activity and calcium deposition studies to show improved osteogenic differentiation on 24-hour NaOH treated scaffolds compared to other groups, indicating their suitability for further studies in bone formation with osteogenic cells.
In this way, through extensive experiments, Zamani et al showed that 24-hour NaOH treated 3-D constructs increased pre-osteoblast proliferation and matrix deposition alongside increased osteogenic activity in BTE. The study demonstrated the potential of the optimized material-surface modification to promote bone formation in lab by facilitating the growth and differentiation of osteogenic cells.
More information: Yasaman Zamani et al. Enhanced osteogenic activity by MC3T3-E1 pre-osteoblasts on chemically surface-modified poly(ε-caprolactone) 3D-printed scaffolds compared to RGD immobilized scaffolds, Biomedical Materials (2018). DOI: 10.1088/1748-605X/aaeb82
Livia Roseti et al. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives, Materials Science and Engineering: C (2017). DOI: 10.1016/j.msec.2017.05.017
Huina Zhang et al. The interaction between bone marrow stromal cells and RGD-modified three-dimensional porous polycaprolactone scaffolds, Biomaterials (2009). DOI: 10.1016/j.biomaterials.2009.04.015
Journal information: Biomaterials
© 2018 Science X Network