Scientists direct bacteria with expanded genetic code to evolve extreme heat tolerance
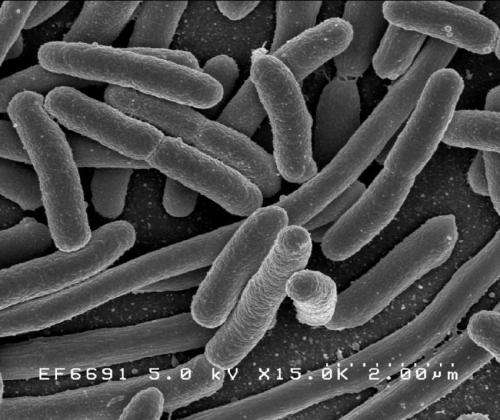
In recent years, scientists have engineered bacteria with expanded genetic codes that produce proteins made from a wider range of molecular building blocks, opening up a promising front in protein engineering.
Now, Scripps Research scientists have shown that such synthetic bacteria can evolve proteins in the laboratory with enhanced properties using mechanisms that might not be possible with nature's 20 amino acid building blocks.
Exposing bacteria with an artificially expanded genetic code to temperatures at which they cannot normally grow, the researchers found that some of the bacteria evolved new heat-resistant proteins that remain stable at temperatures where they would typically inactivate. The researchers reported their findings in the Journal of the American Chemical Society (JACS).
Virtually every organism on earth uses the same 20 amino acids as the building blocks to make proteins—the large molecules that carry out the majority of cellular functions. Peter Schultz, Ph.D., the senior author of the JACS paper and president and CEO of Scripps Research, pioneered a method to reprogram the cell's own protein biosynthetic machinery to add new amino acids to proteins, termed non-canonical amino acids (ncAAs), with chemical structures and properties not found in the common 20 amino acids.
This expanded genetic code has been used in the past to rationally design proteins with novel properties for use as tools to study how proteins work in cells and as new precision-engineered drugs for cancer. The researchers now asked whether synthetic bacteria with expanded genetic codes have an evolutionary advantage over those that are limited to 20 building blocks—is a 21 amino acid code better than a 20 amino acid code from an evolutionary fitness perspective?
"Ever since we first expanded the range of amino acids that can be incorporated in proteins, much work has gone into using these systems to engineer molecules with new or enhanced properties," says Schultz. "Here, we've shown that combining an expanded genetic code with a laboratory evolution one can create proteins with enhanced properties that may not be readily achievable with nature's more limited set."
The scientists started by tweaking the genome of E. coli so that the bacteria could produce the protein homoserine o-succinyltransferase (metA) using a 21 amino acid code instead of the common 20 amino acid code. An important metabolic enzyme, metA dictates the maximum temperature at which E. coli can thrive. Above that temperature, metA begins to inactivate and the bacteria die. The researchers then made mutants of metA, in which almost any amino acid in the natural protein could be replaced with a 21st noncanonical amino acid.
At this point, they let natural selection—the central mechanism of evolution—work its magic. By heating the bacteria to 44 degrees Celsius—a temperature at which normal metA protein cannot function, and as a consequence, bacteria cannot grow—the scientists put selective pressure on the bacteria population. As expected, some of the mutant bacteria were able to survive beyond their typical temperature ceiling, thanks to possessing a mutant metA that was more heat stable—all other bacteria died.
In this way, the researchers were able to drive the bacteria to evolve a mutant metA enzyme that could withstand temperatures 21 degrees higher than normal, nearly twice the thermal stability increase that people typically achieve when restricted to mutations limited to the common 20 amino acid building blocks.
The researchers then identified the specific genetic sequence change that resulted in the mutant metA and found it was due to the unique chemical properties of one of their noncanonical amino acids that laboratory evolution exploited in a clever way to stabilize the protein.
"It's striking how making such a small mutation with a new amino acid not present in nature leads to such a significant improvement in the physical properties of the protein," says Schultz.
"This experiment raises the question of whether a 20 amino acid code is the optimal genetic code—if we discover life forms with expanded codes will they have an evolutionary advantage, and what would we be like if God had worked on the seventh day and added a few more amino acids to the code?"
More information: Jack C. Li et al, Enhancing Protein Stability with Genetically Encoded Noncanonical Amino Acids, Journal of the American Chemical Society (2018). DOI: 10.1021/jacs.8b07157
Journal information: Journal of the American Chemical Society
Provided by The Scripps Research Institute