Metallic nanocatalysts imitate the structure of enzymes

An international team of researchers has transferred certain structural characteristics of natural enzymes, which ensure particularly high catalytic activity, to metallic nanoparticles. The desired chemical reaction thus did not take place at the particle surface as usual, but in channels inside the metal particles – and with three times higher catalytic activity. A team from the University of New South Wales, Australia, and Ruhr-Universität Bochum, Germany, reported on these nanozymes in the Journal of the American Chemical Society, published online on 23 September 2018.
In the case of enzymes, the active centres, where the chemical reaction takes place, are located inside. The reacting substances have to pass through a channel from the surrounding solution to the active centre, where the spatial structure provides particularly favourable reaction conditions. "It is assumed, for example, that a locally altered pH value prevails in the channels and that the electronic environment in the active centres is also responsible for the efficiency of natural enzymes," says Professor Wolfgang Schuhmann, head of the Bochum Center for Electrochemical Sciences.
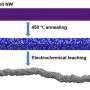
Channels produced in nickel-platinum particles
In order to artificially imitate the enzyme structures, the researchers produced particles of nickel and platinum about ten nanometres in diameter. They then removed the nickel by means of chemical etching, whereby channels were formed. In the final step, they deactivated the active centres on the particle surface. "This enabled us to ensure that only the active centres in the channels participated in the reaction," explains Patrick Wilde, a doctoral candidate at the Center for Electrochemical Sciences. The researchers compared the catalytic activity of the particles produced in this way with the activity of conventional particles with active centres on the surface.
For the test, the team used the oxygen reduction reaction, which, among other things, forms the basis of the operation of fuel cells. Active centres at the end of the channels catalysed the reaction three times more efficiently than active centres on the particle surface.
"The results show the enormous potential of nanozymes," sums up Dr. Corina Andronescu, a group leader at the Center for Electrochemical Sciences. The researchers now want to extend the concept to other reactions, such as electrocatalytic CO2 reduction, and investigate the principles of increased activity in more detail. "We would like to be able to imitate the way enzymes work even better in the future," adds Schuhmann. "Ultimately, we hope that the concept will contribute to industrial applications in order to make energy conversion processes more efficient using electricity generated from renewable sources."
More information: Tania M. Benedetti et al. Electrocatalytic Nanoparticles That Mimic the Three-Dimensional Geometric Architecture of Enzymes: Nanozymes, Journal of the American Chemical Society (2018). DOI: 10.1021/jacs.8b08664
Journal information: Journal of the American Chemical Society
Provided by Ruhr-Universitaet-Bochum