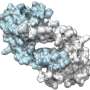
Markus Piotrowski in front of a collage; an image of nitrilase helices taken with an electron microscope is shown at the bottom, a reconstruction of the spiral (calculated from the electron-microscope image) on the right, and the model of a single nitrilase enzyme on the left. Credit: RUB, Marquard
A research team from Ruhr-Universität Bochum (RUB) and from South Africa has analysed two enzymes with identical substrate binding pockets that nevertheless convert different substrates. In the process, it emerged that changes to the enzyme surface affect its substrate specificity by modifying how densely it is packed inside. These findings might pave the way for manipulating the enzyme function. The researchers published their report in the journal Communications Biology on 2 November 2018.
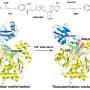
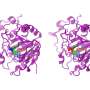
The researchers found that plant enzymes, so-called nitrilases, are very similar. They were able to replace their components piece by piece. "We have thus found that merely by swapping one single component on the surface, we could make one enzyme convert the substrate of another enzyme," explains Associate Professor Dr. Markus Piotrowski from the Department of Molecular Genetics and Physiology of Plants at RUB.
The researchers deployed electron microscopy to analyse why a modification of the surface can affect the substrate binding inside. The analysed nitrilases form larger helices that are big enough to be rendered visible under an electron microscope. "We could thus see that changes to the surface resulted in enzyme molecules in the helix to be more or less densely packed," says Piotrowski. "This, in turn, presumably causes the substrate binding site to be compressed more or less tightly." In its more tightly compressed state, the binding pocket is no longer accessible to larger substrate molecules.
For researchers, nitrilases constitute a model of the evolution of enzymes, but they are also deployed in the chemical and pharmaceutical industry as biocatalysts. To date, experiments aiming at modifying these enzymes by altering their substrate binding site have mostly been unsuccessful. "Our results have shown that the quaternary structure, namely the number and arrangement of individual enzyme molecules, has to be taken into consideration," says Markus Piotrowski. Accordingly, targeted modifications of the enzyme function may be accomplished without performing any changes to the enzyme itself, but merely by compressing it into nitrilase helices with different densities.
More information: Jeremy D. Woodward et al, Substrate specificity of plant nitrilase complexes is affected by their helical twist, Communications Biology (2018). DOI: 10.1038/s42003-018-0186-4
Provided by Ruhr-Universitaet-Bochum