The culprit of superconductivity in cuprates
When it comes to high-temperature superconductors, "high" is a relative term. In the field of superconductivity, "high temperature" means anything that can still be superconductive over 30 degrees Kelvin (K), or a balmy -405 degrees Fahrenheit (F).
The first high-temperature superconductor was discovered in 1986, in ceramic compounds of copper and oxygen known as cuprates. These materials could reach superconductivity around 35 degrees Kelvin or -396.67 degrees Fahrenheit. In the following decades, that temperature limit increased and, to date, researchers have achieved superconductivity in cuprates at temperatures up to 135 degrees Kelvin.
It's important progress, to be sure, but room-temperature superconductivity, which requires operation at 300 degrees Kelvin, is still a long way off, if not impossible.
One of the biggest obstacles is that researchers still don't understand the complete underlying mechanisms of cuprate superconductivity and why there is such variability in superconducting transition temperature among cuprate compounds.
Now, researchers at the Harvard John A. Paulson School of Engineering and Applied Sciences( SEAS) may have the answer. The researchers, led by Xin Li, Assistant Professor of Materials Science at SEAS, found that the strength of a particular chemical bond in cuprate compounds impacts the temperature at which the material achieves superconductivity.
The research is published in Physical Review Letters.
"This could be a new start for designing materials with high-temperature superconductivity," said Li. "Our research sheds lights on a key component of the complicated phenomena in cuprates and points us in a new and exciting direction for materials design."
All cuprates have the same structural building blocks – layered planes of copper peroxide (CuO2) with an out-of-plane oxygen ion, known as the apical oxygen. This oxygen ion sits above each copper atom in the CuO2 plane, like a buoy on the surface of water. The key difference between cuprate compounds comes from what other element is attached to the oxygen buoy. This element is known as the apical cation and can be a variety of elements including lanthanum, bismuth, copper, or mercury.
The temperature at which the material becomes superconductive changes depending on which element is used, but no one really knows why.
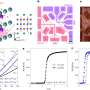
By comparing simulation and experiments, Li and his team demonstrated that the key is the bond between the apical cation and the apical oxygen—the stronger the chemical bond, the higher temperature at which the material becomes superconductive.
But why does this bond raise superconductive temperatures?
Superconductors are often described as electron superhighways, or super carpool lanes, in which paired electrons are cars and the superconducting material is the special, frictionless road for the cars to move.
However, electrons don't really move across a high-temperature superconductor like a car on a road. Instead, they hop. This hopping process is made a lot easier when the crystal lattice on which the electrons are moving oscillates in a particular way.
A strong chemical bond between the apical anion and apical cation increases the oscillation of both the lattice and the induced electric current.
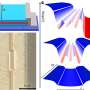
Imagine a kite tied to a buoy and many such kite-buoy units line up. If the bond between the kite and the buoy is strong, the kite can pull the buoy up and down, causing ripples and splashes in the water. The ripples are akin to the lattice oscillation and the splashes represent the electrons that get pushed out of the CuO2 plane. The ripples and splashes are not chaotic, rather, they cooperatively follow certain rules that tell the bouys how to oscillate in the best way to help the electron hop easily along the material.
"We demonstrated that this structural unit—the copper oxygen layer, the apical anion, and the apical cation—is a fundamental building block that can couple dynamically to control the superconductive properties of the material," said Li. "This opens up an entirely new avenue to explore the superconductive properties of materials."
Next, the researchers aim to explore how this novel effect impacts our understanding of the mysterious phase diagram in high-temperature superconductors, including the pairing mechanism in these superconductors.
More information: Apical Charge Flux-Modulated In-Plane Transport Properties of Cuprate Superconductors. Phys. Rev. Lett. doi.org/10.1103/PhysRevLett.121.157001
Journal information: Physical Review Letters
Provided by Harvard University