Synthesis studies transform waste sugar for sustainable energy storage applications
Biorefinery facilities are critical to fueling the economy—converting wood chips, grass clippings, and other biological materials into fuels, heat, power, and chemicals.
A research team at the US Department of Energy's (DOE's) Oak Ridge National Laboratory has now discovered a way to create functional materials from the impure waste sugars produced in the biorefining processes.
Using hydrothermal carbonization, a synthesis technique that converts biomass into carbon under high temperature and pressure conditions, the team transformed waste sugar into spherical carbon materials. These carbon spheres could be used to form improved supercapacitors, which are energy storage devices that help power technologies including smartphones, hybrid vehicles, and security alarm systems. The team's results are published in Scientific Reports, a Nature research journal.
"The significant finding is that we found a way to take sugar from plants and other organic matter and use it to make different structures," said Amit Naskar, a senior researcher in ORNL's Materials Science and Technology Division. "Knowing the physics behind how those structures form can help us improve components of energy storage."
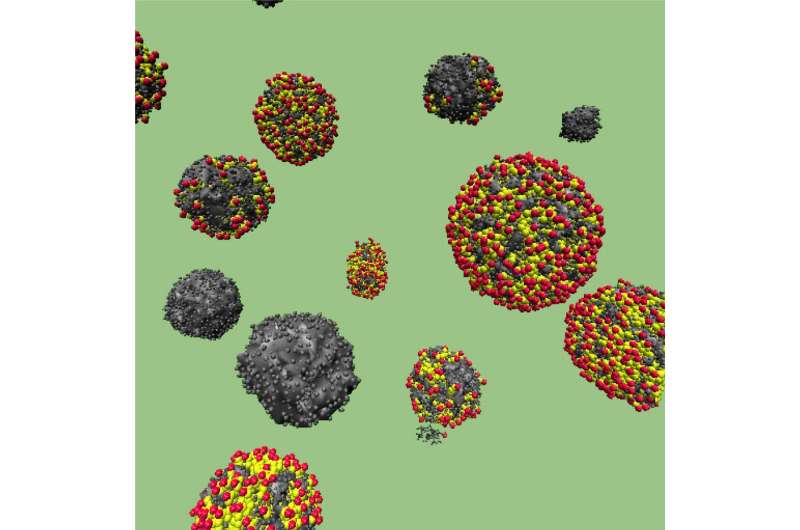
By modifying the synthesis process, the researchers created two varieties of the novel carbon spheres. Combining sugar and water under pressure resulted in solid spheres, whereas replacing water with an emulsion substance (a liquid that uses chemicals to combine oil and water) typically produced hollow spheres instead.
"Just by substituting water for this other liquid, we can control the shape of the carbon, which could have huge implications for supercapacitor performance," said Hoi Chun Ho, a Ph.D. candidate working with Naskar at the Bredesen Center for Interdisciplinary Research and Graduate Education, a joint venture of ORNL and the University of Tennessee, Knoxville. The team also discovered that altering the duration of synthesis directly affected the size and shape of the spheres.
To further explore the discrepancies between solid and hollow carbon structures, the team ran synthesis simulations on the Cray XK7 Titan supercomputer at the Oak Ridge Leadership Computing Facility (OLCF), a DOE Office of Science User Facility located at ORNL. They also used transmission electron microscopy (TEM) and small-angle X-ray scattering (SAXS) tools at the Center for Nanophase Materials Sciences (CNMS), another DOE Office of Science User Facility, to characterize the capabilities and structure of the carbon samples.
"We wanted to determine what kind of surface area is good for energy storage applications, and we learned that the hollow spheres are more suitable," said ORNL researcher Monojoy Goswami of CNMS and the Computer Science and Engineering Division. "Without these simulations and resources, we wouldn't have been able to reach this fundamental understanding."
With this data the team tested a supercapacitor with electrodes made from hollow carbon spheres, which retained about 90 percent capacitance—the ability to store an electric charge—after 5,000 charge cycles. Although supercapacitors cannot store as much energy as batteries can store, they have many advantages over batteries, such as faster charging and exceptionally long lifetimes. Some technologies contain both batteries to provide everyday energy and supercapacitors to provide additional support during peak power demands.
"Batteries often support smartphones and other electronic devices alone, but supercapacitors can be useful for many high-power applications," Ho said. "For example, if a vehicle is driving up a steep hill with many passengers, the extra strain may cause the supercapacitor to kick in."
The pathway from waste sugar to hollow carbon spheres to supercapacitors demonstrates new potential for previously untapped byproducts from biorefineries. The researchers are planning projects to find and test other applications for carbon materials derived from waste sugar such as reinforcing polymer composites with carbon fibers.
"Carbon can serve many useful purposes in addition to improving supercapacitors," Ho said. "There is more work to be done to fully understand the structural evolution of carbon materials."
Making use of waste streams could also help scientists pursue forms of sustainable energy on a broader scale. According to the ORNL team, biorefineries can produce beneficial combinations of renewable energy and chemicals but are not yet profitable enough to compete with traditional energy sources. However, the researchers anticipate that developing useful materials from waste could help improve efficiency and reduce costs, making outputs from these facilities viable alternatives to oil and other fossil fuels.
"Our goal is to use waste energy for green applications," Goswami said. "That's good for the environment, for the biorefinery industry, and for commerce."
More information: Hoi Chun Ho et al. Amending the Structure of Renewable Carbon from Biorefinery Waste-Streams for Energy Storage Applications, Scientific Reports (2018). DOI: 10.1038/s41598-018-25880-0
Journal information: Scientific Reports , Nature
Provided by Oak Ridge National Laboratory




















