Highly efficient single-atom catalyst could help auto industry
A longer-lasting, higher-efficiency platinum catalyst has been developed by a Dalhousie University-led team, a result with major implications for the automobile industry.
Platinum catalysts help deactivate toxic exhaust gases from traditional car engines. Platinum is also used to help drive the chemical reactions that make zero-emissions hydrogen fuel cells possible—a technology that could transform automobiles as we know them.
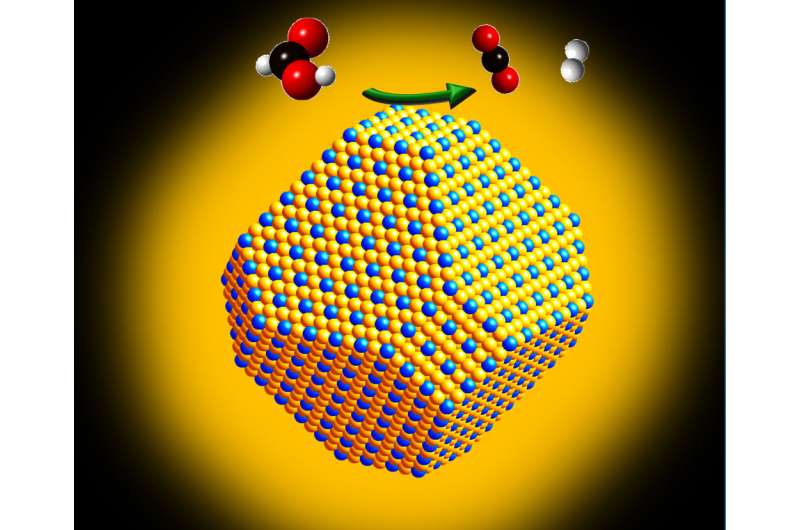
The new catalyst combines gold and platinum to form what's known as a single-atom catalyst, resulting in nearly 100-fold increases in efficiency over market platinum catalysts, says Peng Zhang, the Dalhousie professor who led this research.
Not only is efficiency improved at the outset, but it is maintained through the catalyst's lifetime: normally, a platinum catalyst works less well over time as carbon monoxide molecules tightly bond to and block platinum from helping reactions along.
Improvements come from two properties: the single atom structure, which maximizes platinum's active surface area, and the unique electronic properties that adding gold to create an alloy helps to achieve.
"The magic happens because of the alloy. Think about iron: it very easily gets rusty in the air, but if you have an iron alloy, like stainless steel, its properties are totally different," says Zhang.
For example, making a gold-platinum alloy stops the platinum catalyst from losing efficiency and "poisoning" over time. Catalyst poisoning is one of the major struggles.
This is because if two platinum atoms are side-by-side, then carbon monoxide from the chemical reaction binds tightly to them, poisoning the catalyst and gradually degrading its efficiency. When researchers ensure that there are no platinum clusters within a larger gold lattice, the poisoning effect disappears.
Zhang's team worked with three Canadian Light Source facilities at the University of Saskatchewan to understand and test their alloyed catalysts, as they tried various combinations and structures of platinum and gold.
"Synchrotrons are one of the most powerful tools to study. Alloys are super hard to study with regular tools, since it's hard to distinguish the two metals from each other," says Zhang. "With a synchrotron, you can easily tune the energy to take only the platinum, and then the gold."
As the researchers reduced the platinum content of their catalyst, they found great improvements in its function, until eventually they hit upon the single-atom model that maximized platinum's surface area and minimized poisoning.
Further, the team found that the simple chemical technique they used to prepare the alloy resulted in a higher overall concentration of platinum atoms than typical alloys by nearly 10 times. Alloys normally contain very low concentrations of single atoms, below 1%. Zhang's team created an alloy with 7% single-platinum atoms.
The work is not yet done, of course. Zhang and his team built this first alloy with gold because it is highly stable, which makes it great for testing new ideas. But of course, like platinum, gold is very expensive.
"Gold is a first step to show the concept. We now want to look at other, less expensive metals, which will make this more usable for industry," says Zhang.
More information: Paul N. Duchesne et al. Golden single-atomic-site platinum electrocatalysts, Nature Materials (2018). DOI: 10.1038/s41563-018-0167-5
Journal information: Nature Materials
Provided by Canadian Light Source