Shining X-ray light on perovskites for better solar cells

Named after a mineral discovered in the Ural Mountains of Russia, perovskites have taken center stage as a class of materials with properties that could be applied to future electronics and energy devices.
Semiconducting films made of perovskites promise flexible, light-weight solar cells that are cheap and easily made from abundant materials. While they are not yet available commercially – hurdles include making them more stable and durable – they may transform the solar energy industry in the next decade or two.
For scientists, perovskites also present an interesting puzzle: Start with any number of variations on the basic ingredients for making them—lead, iodide and methylammonium – and you end up with the same basic material. Yet, tweaks to the chemistry at various stages in the process can lead to perovskites with more desirable qualities for solar cells.
For researchers at the Stanford Synchrotron Radiation Lightsource (SSRL) and Stanford University, the mystery and potential of perovskites converge in experiments where extremely bright X-rays are used to study the chemistry of the material in the very moments it is being formed. The DOE Office of Science user facility at SLAC National Accelerator Laboratory offers multiple ways to approach the problem and discover new insights about this useful material.
We asked SSRL staff scientists Christopher Tassone and Kevin Stone, Stanford Chemistry Ph.D. student Aryeh Gold-Parker and Michael Toney, head of the SSRL materials science division, what they recently found out about perovskite chemistry and where they are hoping their work will lead.
Their research was published today in Nature Communications.
How are perovskites made, and what interests you about this process?
Stone: You start by dissolving some basic ingredients in a solvent. Then you deposit that solution and dry it into a film. The film is then transformed into the final perovskite by a treatment such as annealing, which involves heating it to a certain temperature and then cooling it again. We are interested in the chemistry of that entire process and how it evolves at each stage. The idea is that if you can understand what we call the "formation chemistry" of perovskites, you can create the materials to have the exact properties you desire.
Gold-Parker: There are dozens of different methods for depositing perovskite films, for example. And these methods lead to differences in thickness, texture, grain size and crystallinity of the films. In the lab, creating perovskites with distinctive characteristics is mostly done through trial and error. Engineers make small changes to the process to optimize the particular property they're interested in, whether that's solar cell voltage or performance. Trial and error can work, but it's not efficient.
Tassone: My group is really interested in how we make large quantities of solar panels very cheaply to meet growing demands for solar power and clean energy goals. Conventional silicon solar cells can't be manufactured rapidly enough. We believe that if we can understand the chemical transformations that are occurring during the process of making perovskite solar cells, we can ultimately engineer better processes that meet the needs of industry.
What was your latest study about?
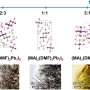
Gold-Parker: Our study builds on work by other groups of researchers at Oxford, Cornell and Stanford that showed using chlorine in the processing can lead to high-quality perovskite films with impressive performance. After the solution is deposited there's an intermediate step where a crystalline film forms – we call this a precursor – and then a gaseous salt of chlorine called methylammonium chloride (MACI) leaves the film continually while it's converting into a perovskite. A few years ago, an SSRL study by myself, Toney and co-workers showed there is very little chlorine left in the final product. Even though you start with quite a lot of chlorine, the vast majority of it is lost in the processing.
Stone: In this latest study we wanted to know: Where does the chlorine go and what purpose does it serve? Why chlorine in the first place? What does the precursor consist of, and how is it influencing this transformation?
What did you find out?
Stone: We were able to figure out what the structure of that crystalline precursor is, how the atoms are put together, and roughly how much chlorine is present. When we heat it up during the annealing stage, we see that crystalline precursor persists for quite a while before it begins to transform into perovskite.
Gold-Parker: We were also able to show that the transformation into the final perovskite is limited by the gradual evaporation of MACl, and that this slow transformation might actually lead to a higher quality perovskite material.
Toney: There are also broader implications. Theory calculations can tell you with good accuracy the properties your material will have. But they provide almost no guidance about how to go about synthesizing it. This question has driven interest in the science community over many decades, but even more so over the last five years, in what's been called synthesis science: understanding how you actually make something. What are the processes that the material goes through, the pathways? This study is one very nice example of being able to disentangle that synthesis process, and as a result gain insight into how we could redesign it.
How did you study it?
Tassone: We used multiple versions of two techniques called X-ray scattering and X-ray spectroscopy. X-ray scattering is used to study structure; it tells you where the atoms are located in crystalline materials. X-ray spectroscopy is a complementary technique. It tells you about the chemistry of the film, how much of the different chemical elements are present and how they are bonded.
Gold-Parker: These methods allowed us to probe changes in the crystal structure and the amount of chlorine throughout the transformation, as well as the chemical state of the chlorine. And very importantly, we used each of those techniques in situ – or as the changes are actually occurring. SSRL has world-class capabilities for designing and performing these sorts of in situ experiments that monitor the actual process instead of just the starting and end points, and that was really powerful.
Tassone: What makes this result and our approach very strong is that we use the interpretation of the scattering data to inform the interpretation of the spectroscopy data, and vice versa. We would not have solved this mechanism without moving those things together. In the paper we lay out a clear pathway for anyone who wants to study the processes involved in making this or other materials. This is an important step in perovskites research but also in the broader field of synthesis science that Mike described.
What's next?
Stone: I would like to study what happens in the solution before it dries, so at an earlier stage in the process. I would also like to expand our methods to include other perovskite materials.
Toney: Another point to pursue is related to the role of the chlorine that's present in the film in this specific example. It serves as a mediator or regulator, and it slows down the conversion. How does this general concept of a mediator – a compound that serves a purpose but does not end up in your final material – work in this process or other processes or materials? Silicon has been studied for at least 50 years, perovskites for five, so we've got a lot of work ahead of us.
Tassone: I have two points for moving forward. One is how do we develop the processes that will work at scale and allow solar to be affordable to everyone and really make a large impact on our energy landscape? The other is, based on the fact that perovskites are the most exciting semiconductor development in the last decade or two, how can we utilize the unique properties of this material for other applications as well?
More information: Kevin H. Stone et al. Transformation from crystalline precursor to perovskite in PbCl2-derived MAPbI3, Nature Communications (2018). DOI: 10.1038/s41467-018-05937-4
Journal information: Nature Communications
Provided by SLAC National Accelerator Laboratory