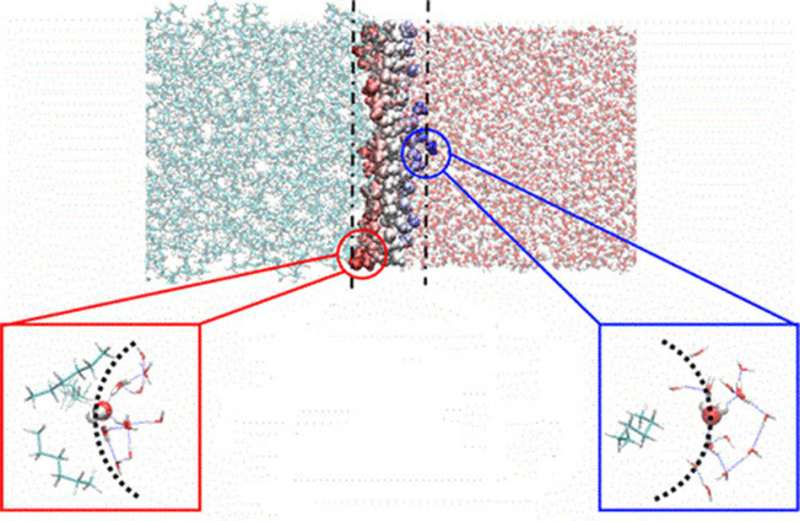
How molecules move and organize in water when the liquid meets the oil depends on whether or not the chemical is on the crest or trough of a tiny wave of water at the interface. Credit: US Department of Energy
Oil and water don't mix. The interface between the two liquids is a building block of the most widely used industrial process to purify chemicals in energy production (from nuclear energy to biofuels) and storage (batteries). A recent discovery may unlock mechanisms that transport molecules across the boundary between oil and water. Simulations and analyses reveal a highly diverse and dynamic structure at the interface. In fact, the crests of tiny waves of water into the oil phase cause water to behave as if it is a gas—potentially easing transport of chemicals from water to oil.
Extracting a chemical from water and moving it into an oil (solvent extraction) is a mature technology. However, solvent extraction hasn't seen major advancement in many decades. The molecules at the interface are the gatekeepers. Knowing how those molecules organize and move is the first step toward designing interfaces that precisely control transport and reactivity. This work is analogous to tailored solid:liquid interface technologies that have revolutionized applications in catalysis. Solid:liquid interfaces also have changed materials synthesis and science.
Decades of research have empirically shown optimal conditions for a variety of separations applications, making use of specific combinations of solvents, chemical extractant molecules, and pH for a successful process. Yet the essential role of the interface has remained elusive, in part because even advanced molecular dynamics simulations have focused on its average behavior. New data analytics approaches of simulation data (developed at Washington State University and run at the Oak Ridge Leadership Computing Facility) have quantified the heterogeneity of the crests and troughs of the surface capillary waves—revealing extreme differences of behavior of the solvents as they ride the wave in time. The crests of waves are characterized by protrusions where water behaves as if it is a vapor (less hydrogen bonding, slower water rotational dynamics) —a feature that increases in the presence of solutes and may explain water extraction into the organic phase, a well-observed behavior in many conditions.
More information: Tiecheng Zhou et al. Structural and Dynamic Heterogeneity of Capillary Wave Fronts at Aqueous Interfaces, The Journal of Physical Chemistry B (2017). DOI: 10.1021/acs.jpcb.7b07406
Journal information: Journal of Physical Chemistry B
Provided by US Department of Energy