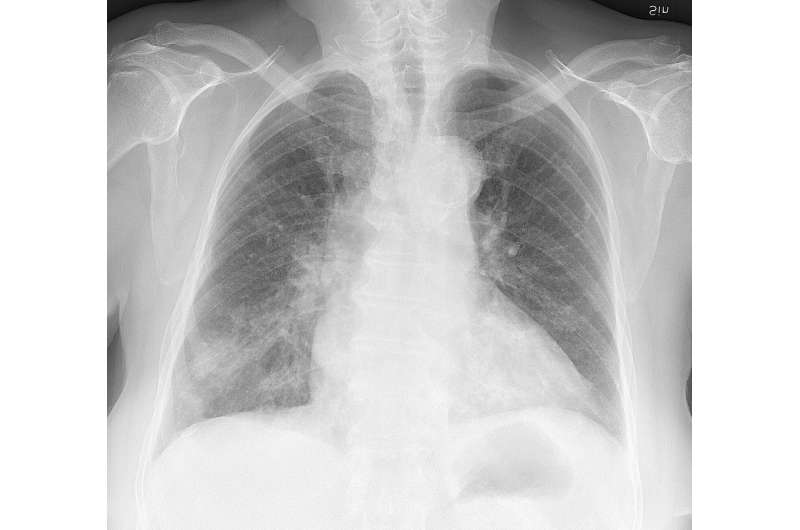
Chest X-ray of a 76 year old woman, who developed cough and labored breathing. First testing showed influenza B virus, and later a nasopharyngeal swab detected Haemophilus influenzae. The H influenzae presumably developed as an opportunistic infection secondary to the flu. This X-ray was taken 2 weeks after cultures and start of antibiotics, showing delayed pneumonic infiltrates that were only vaguely visible on initial (not shown) X-rays. Credit: Mikael Häggström
Successful pathogenic strains of pneumococci have two proteins that, owing to their location on the surface of the bacteria, enhance their survival and ability to cause disease, according to a study from Karolinska Institutet in Sweden published in Nature Communications .
Pneumococcal infections are one of the most common causes of disease and death in the world. One reason for the pathogenic potential of these bacteria is that they produce a sugar casing. This capsule prevents the important immune component C3b from attaching to and attacking the bacteria.
Researchers at Karolinska Institutet and the Royal Institute of Technology in Sweden have now studied in detail how pneumococci interact with the part of the immune system called the complement system, which includes C3b. The complement system often works as the first line of defence against foreign substances and cells, triggering a number of immune reactions in the body.
The researchers show that the capsule is weak at the bacteria's point of division, which therefore presents an opening for C3b. By using super-resolution microscopy (STED) they found that C3b accumulates under the capsule primarily at the division sites. This accumulation can continue and cover the entire bacteria unless the pneumococcus can find a strategy to prevent it happening.
The study also shows that a common surface protein on pneumococci called PspC1 is located right at the division site, where it recruits another protein called Factor H, which negatively regulates the complement system by, amongst other mechanisms, inactivating C3b. Some especially successful and pathogenic pneumococcal strains also express a closely related protein, PspC2, which is mainly localised at the bacterial poles. This separate location on the surface of the bacteria affects the two surface proteins' functions. Unlike PspC1, which binds Factor H, PspC2 affects the bacteria's ability to adhere to epithelial cells, which can be found in the respiratory tract, in mucus membranes and elsewhere.
"Our study shows that the precise localisation of bacterial surface proteins in relation to the capsule layer affects the role they will have in the disease development," says Birgitta Henriques-Normark, professor at the Department of Microbiology, Tumour and Cell Biology, Karolinska Institutet. "This is an important piece of the puzzle to understand how pneumococci avoid the immune system and cause everything from otitis and sinusitis to severe pneumonia and septicaemia."
More information: Anuj Pathak et al. Factor H binding proteins protect division septa on encapsulated Streptococcus pneumoniae against complement C3b deposition and amplification, Nature Communications (2018). DOI: 10.1038/s41467-018-05494-w
Journal information: Nature Communications
Provided by Karolinska Institutet