Recording every cell's history in real-time with evolving genetic barcodes
All humans begin life as a single cell that divides repeatedly to form two, then four, then eight cells, all the way up to the ~26 billion cells that make up a newborn. Tracing how and when those 26 billion cells arise from one zygote is the grand challenge of developmental biology, a field that has so far only been able to capture and analyze snapshots of the development process.
Now, a new method developed by scientists at the Wyss Institute and Harvard Medical School (HMS) finally brings that daunting task into the realm of possibility using evolving genetic barcodes that actively record the process of cell division in developing mice, enabling the lineage of every cell in a mouse's body to be traced back to its single-celled origin.
The research is published today in Science as a First Release article.
"Current lineage-tracking methods can only show snapshots in time, because you have to physically stop the development process to see how the cells look at each stage, almost like looking at individual frames of a motion picture," said senior author George Church, Ph.D., who is a Founding Core Faculty member at the Wyss Institute, Professor of Genetics at HMS, and Professor of Health Sciences and Technology at Harvard and MIT. "This barcode recording method allows us to reconstruct the complete history of every mature cell's development, which is like playing the full motion picture backwards in real-time."
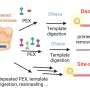
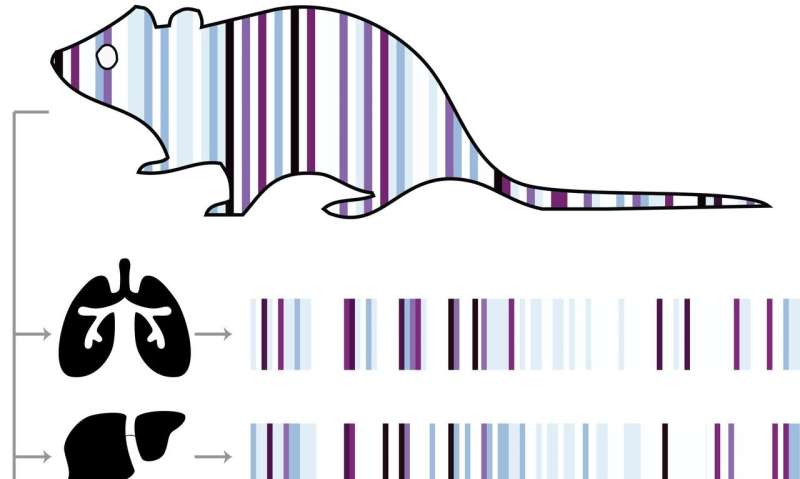
The genetic barcodes are created using a special type of DNA sequence that encodes a modified RNA molecule called a homing guide RNA (hgRNA), which was developed in a previous paper. hgRNA molecules are engineered such that when the enzyme Cas9 (of CRISPR-Cas9 fame) is present, the hgRNA will guide the Cas9 to its own hgRNA sequence in the genome, which Cas9 then cuts. When the cell repairs that cut, it can introduce genetic mutations in the hgRNA sequence, which accumulate over time to create a unique barcode.
The researchers implemented the hgRNA-Cas9 system in mice by creating a "founder mouse" that had 60 different hgRNA sequences scattered throughout its genome. They then crossed the founder mouse with mice that expressed the Cas9 protein, producing zygotes whose hgRNA sequences started being cut and mutated shortly after fertilization.
"In every single cell that the zygote divides to become, there's a chance that its hgRNAs will mutate," explained first author Reza Kalhor, Ph.D., a postdoctoral research fellow at the Wyss Institute and HMS. "In each generation, all the cells acquire their own unique mutations in addition to the ones they inherit from their mother cell, so we can trace how closely related different cells are by comparing which mutations they have."
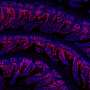
Each hgRNA can produce hundreds of mutant alleles; collectively, they can generate a unique barcode that contains the full developmental lineage of each of the ~10 billion cells in an adult mouse.
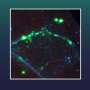
The ability to continuously record cells' development also allowed the researchers to resolve a longstanding question regarding the embryonic brain: does it distinguish its front from its back end first, or its left from its right side first? By comparing the hgRNA mutation barcodes present in cells taken from different parts of two mice's brains, they found that neurons from the left side of each brain region are more closely related to neurons from the right side of the same region than to neurons from the left side of neighboring regions. This result suggested that front-back brain patterning emerges before left-right patterning in the development of the central nervous system.
"This method allows us to take the final developmental stage of a model organism and from there reconstruct a full lineage tree all the way back to its single-cell stage. It's an ambitious goal that will certainly take many labs several years to realize, but this paper represents an important step in getting there," said Church. The researchers are now focusing on improving their readout techniques so that they can analyze the barcodes of individual cells and reconstruct the lineage tree that has been recorded.
"Being able to record cells continuously over time is a huge milestone in developmental biology that promises to exponentially increase our understanding of the process by which a single cell grows to form to an adult animal and, if applied to disease models, it could provide entirely new insights into how diseases, such as cancer, emerge," said Donald Ingber, M.D., Ph.D., Founding Director of the Wyss Institute who is also the Judah Folkman Professor of Vascular Biology at HMS and the Vascular Biology Program at Boston Children's Hospital, as well as Professor of Bioengineering at Harvard's John A. Paulson School of Engineering and Applied Sciences (SEAS).
More information: R. Kalhor el al., "Developmental barcoding of whole mouse via homing CRISPR," Science (2018). science.sciencemag.org/cgi/doi … 1126/science.aat9804
Journal information: Science
Provided by Harvard University