Blending liquid MOFs produces new glass materials
Metal-organic frameworks (MOFs) are a class of crystalline materials with a structure of inorganic nodes connected by organic ligands. There are currently more than 60,000 known MOFs, and they are being investigated as promising materials for gas storage, including CO2 sequestration and hydrogen storage, and can even be used to harvest water in the desert. Research so far has concentrated on MOFs in the solid state, but these soft, microcrystalline powders are hard to process industrially, as they can't be sintered and are hard to form into pellets.
Curiously, several examples of the melting of crystalline MOF structures have been observed, along with the formation of a liquid of identical composition to the parent framework. Cooling of these liquids then results in a new family of gasses. The fundamental novelty of the liquid and glass MOF states, compared with their more widely known crystalline states, provoked early research1 into the reactivity of the liquid state, and particularly how a liquid MOF may interact with another MOF component.
To investigate, an international team of researchers brought a pair of crystalline MOFs (ZIF-4 and ZIF-62) to Diamond, carrying out research on their behaviour during heating, on the I22 and I15-1 beamlines. Their results, recently published in Nature Communications, show that the MOF liquid state can be blended with another MOF component to form a MOF glass with a tailorable glass transition.
Using new techniques to investigate amorphous MOFs
Dr. Tom Bennett is a materials chemist. The research of his group is focused on the synthesis, characterization and application of non-crystalline MOFs, and particularly MOF-liquids and MOF-glasses. His aims are twofold: to diversify MOF research away from a pure focus on the crystalline state, and to explore the interfaces of the field with glasses, ionic liquids, and polymers. In this research, his team used in situ experiments at Diamond to investigate two MOF components melted together, tracked with temperature.
Investigating how two amorphous MOF components interact is incredibly complex, requiring new techniques, and the research group have used the I15-1 beamline before, to produce pair distribution functions (PDFs) of their materials. A PDF indicates distances over which two atoms are separated on a repeating scale, regardless of whether the material is crystalline or not.
The idea of using small and wide angle X-ray scattering (SAXS and WAXS) on these materials was first raised by I22 beamline scientist Dr. Andy Smith.
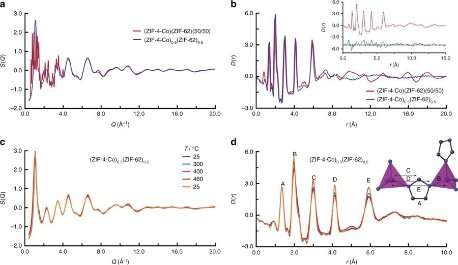
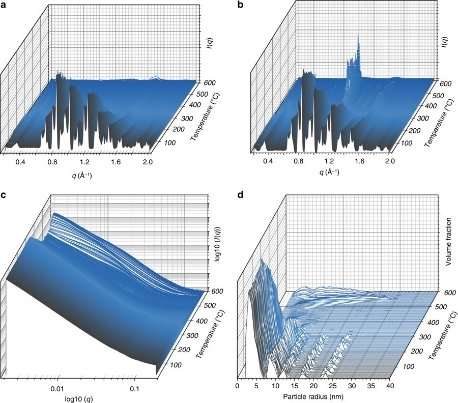
In-situ SAXS and WAXS measurements were taken during heating of the two MOFs together, and demonstrated formation of a liquid from one component, and an amorphous solid from the other. The measurements also demonstrated coalescence of particles together, confirming, along with differential scanning calorimetry results, that the two MOF components had blended together – in a manner known in the organic polymer world, but not in MOFs. XPDF measurements were used on the recovered 'MOF-blends', and confirmed the presence of the metal-ligand connectivity associated with the MOF state.
Dr. Smith explains:
Unlike with many of Diamond's techniques we cannot see individual atoms with SAXS since the technique works at longer length scales, those of large molecules or molecular assemblies. In this work we were able use SAXS to watch the changes occurring as the microcrystals of MOF melted into the glassy state and correlate this with simultaneous WAXS data showing the gradual loss of crystallinity. Combination of SAXS/WAXS on I22 with PDF measurements on I15-1 allows a fuller understanding of these complex materials under processing conditions. It is an area we hope to do a lot more work in."
Another innovation was to use ex situ energy-dispersive X-ray spectroscopy (EDS) to give element-specific results, and EDS tomography. The resulting 3-D images show that the two MOF phases bind across the interface between them, in a ligand swapping process. The two MOFs used in these experiments were quite viscous; further experiments showed that starting with smaller particles of the crystalline MOFs resulted in a greater degree of mixing, or blending.
What's next for liquid MOFs?
The next steps for this research are to investigate which MOFs can be blended together to make useful new materials.
Dr. Bennett feels that there's still a lot to learn about amorphous MOFs. "MOF-glasses and MOF-liquids have so much potential, and there is a lot of room for input from other fields, including glasses, ionic liquids, and polymers. It's likely that many other researchers have formed amorphous or liquid MOFs whilst searching for a new crystal structure, but then thrown them away. In reality, these non-crystalline states may turn out to be just as, if not more, interesting than the crystals themselves. The field is only really just getting started, and I'm very interested in collaborating with researchers from other domains," he says. He finds Twitter a useful tool, both for collaboration and for engaging with people generally.
More information: Louis Longley et al. Liquid phase blending of metal-organic frameworks, Nature Communications (2018). DOI: 10.1038/s41467-018-04553-6
Journal information: Nature Communications
Provided by Diamond Light Source




















