Materials scientists from Lomonosov Moscow State University explained the laws of dissolution and crystallization of hybrid perovskites and have proposed a novel approach for obtaining films for solar cells. They have explained the key mechanisms of interaction of hybrid perovskites with solvents and suggested new approaches to obtain perovskite light-absorbing layers for thin-film solar cells from weakly coordinating aprotic solvents.
The results of the study have been recently published in the high-rating journal Chemistry of Materials.
Thin-film solar cells based on hybrid perovskites have already reached an efficiency of 23.2 percent, surpassing traditional solar cells based on silicon. The light-absorbing layer of perovskite in such devices can be obtained by simpler and cheaper solution methods. The scientists studied the processes of perovskite crystallization from a solvent with unusual properties—gamma-butyrolactone (GBL).
"In our laboratory we develop new innovative non-solvent methods for obtaining solar cells but also pay great attention to the fundamental aspects of perovskite chemistry. This is a traditional characteristic trait of the materials science school of Lomonosov Moscow State University, which distinguishes us from most of the world's groups," said contributing researcher Alexey Tarasov.
There are two solvents that are usually used to prepare perovskite thin films from solutions: dimethylsulfoxide and dimethylformamide. However, earlier work showed that crystallization from these solvents proceeds through formation of intermediate compounds—crystallosolvates, which can impair the morphology and functional properties of the perovskite layer.
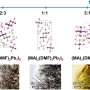
As a solvent for perovskite, GBL exhibits so-called retrograde solubility—the solubility of perovskite in GBL decreases with the increase of temperature. This feature was widely used by researchers to produce single crystals, whereas the attempts to obtain a thin film resulted in the formation of separated individual crystallites on a substrate. For a long time, this unusual behavior of perovskite solutions in GBL remained poorly understood. It was believed that the perovskite-GBL interaction is weak enough that it does not even form solvates with it. However, scientists discovered that there are at least three types of perovskite crystals with GBL, and some of them have a unique cluster structure. It became clear that the equilibrium in perovskite solutions in GBL is much more complicated than previously expected.
"We have established that perovskite dissolves at room temperature with the formation of such clusters, and upon heating, they decompose to small complexes. This leads to supersaturation and precipitation of perovskite from solution in the form of single crystals. We showed that it was the precipitation of a cluster adduct instead of perovskite that prevented the formation of thin films from this solvent. Based on the understanding of the processes that occur during the dissolution of perovskite in GBL, we proposed approaches that bypass the formation of clusters and results in perovskite crystallization. Consequently, we obtained high-quality films from GBL for the first time. This is an excellent example of the practical application of fundamental chemical knowledge for the solution of materials science problems—just what is generally called fundamental material science throughout the world," concluded Alexey Tarasov.
More information: Sergey A. Fateev et al, Solution processing of methylammonium lead iodide perovskite from gamma-butyrolactone: crystallization mediated by solvation equilibrium, Chemistry of Materials (2018). DOI: 10.1021/acs.chemmater.8b01906
Journal information: Chemistry of Materials
Provided by Lomonosov Moscow State University