Credit: American Chemical Society
A cranberry, honey or a candy bar—which tastes the sweetest? These foods contain sugars that humans can perceive differently. A cranberry seems tart, whereas a candy bar can be excessively sweet, and honey is somewhere in the middle. Now, in a study in ACS' The Journal of Physical Chemistry Letters, researchers have shown that the perception of sweetness depends on molecular interactions between specific sugars and water in the saliva.
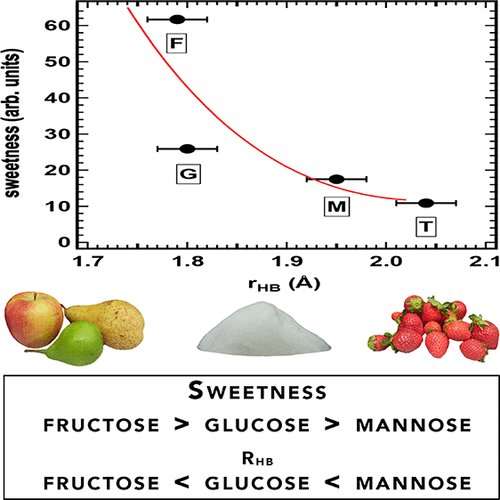
The sugars mannose, glucose and fructose have almost identical chemical structures. Yet fructose (found in many candy bars) is about twice as sweet as glucose (found in honey), whereas mannose (found in cranberries) is considered tasteless. Sugars stimulate specific protein receptors on the taste buds of the tongue, which sends a signal to the brain that a food tastes sweet. But scientists don't know why we perceive some sugars as being sweeter than others. Because these interactions take place in saliva, which is mostly water, Maria Antonietta Ricci and colleagues wondered if water might play a role.
The researchers used a technique called neutron diffraction with isotopic substitution to probe the structures of mannose, glucose and fructose in water. They found that none of the sugars substantially disrupted how water molecules interact with each other. However, the three sugars interacted with water molecules in different ways. Mannose, the least sweet of the sugars, formed longer and weaker hydrogen bonds with water than glucose or fructose. Fructose, the sweetest of the sugars, formed the shortest and strongest hydrogen bonds with water. The researchers surmise that shorter hydrogen bonds with water could allow the sugar molecule to bind more snugly with the protein receptor, causing greater stimulation and perception of sweetness.
More information: F. Bruni et al. Hydrogen Bond Length as a Key To Understanding Sweetness, The Journal of Physical Chemistry Letters (2018). DOI: 10.1021/acs.jpclett.8b01280
Abstract
Neutron diffraction experiments have been performed to investigate and compare the structure of the hydration shell of three monosaccharides, namely, fructose, glucose, and mannose. It is found that despite their differences with respect to many thermodynamical quantities, bioprotective properties against environmental stresses, and taste, the influence of these monosaccharides on the bulk water solvent structure is virtually identical. Conversely, these sugars interact with the neighboring water molecules by forming H bonds of different length and strength. Interestingly, the sweetness of these monosaccharides, along with that of the disaccharide trehalose, is correlated with the length of these H bonds. This suggests that the small differences in stereochemistry between the different sugars determine a relevant change in polarity, which has a fundamental impact on the behavior of these molecules in vivo.
Journal information: Journal of Physical Chemistry Letters
Provided by American Chemical Society