Baker's yeast helps biologists to understand drug resistance in fungi
MSU-based biologists have reported new understanding of fungal drug resistance mechanisms. To study them, the scientists used baker's yeast expressing fluorescent proteins fused with the membrane transporters. Fungi activate the protection mechanisms in response to accumulation of toxic compounds in their cells. The study showed that the resistance of yeast (and possibly pathogenic fungi) to antimycotic agents is more complex than it was previously thought. Its results were published in the Scientific Reports journal.
Drug resistance of pathogenic microorganisms is a serious medical problem. The most widely known threat is antibiotic-resistant bacteria. Some pathogenic fungi is also evolved to activate mechanisms of protection against antifungal agents. One of such mechanisms is hyperactivation of ABC-transporters. These transporter proteins are localized in the plasma membranes of and extrude potentially harmful substances from the cytoplasm.
In fungi with hyperactivated multiple drug resistance, including pathogenic ones, ABC-transporters extrude antimycotics, thus considerably decreasing the efficiency of the treatments against fungal infections. In 2016, a team from Belozersky Institute of Physico- Chemical Biology proposed a method of suppressing multiple drug resistance in fungi. The scientists suppressed the protection mechanism of yeast cells using alkyl-rhodamines—compounds that on their own are not harmful for microorganisms. Like other lipophilic cations (positively charged ions that are moderately soluble in water), these substances easily pass through cell membranes. Moreover, alkyl-rhodamines are fluorescent and are therefore often used in the laboratory studies as dyes.
Alkyl-rhodamines induced the futile activity of the ABC-transporters: Instead of discarding toxic antimycotics, they started to pump out the dye. However, after being transported out of the cells, lipophilic cations tend to return instantly. As a result, the majority of ABC-transporters were busy pumping out the harmless dyes, while the antimycotic agent added simultaneously with alkyl-rhodamines was accumulating in the cytoplasm.
"When the concentration of a xenobiotic in a cell increases, it initiates a compensatory response, i.e. the cell starts to produce more ABC-transporter proteins to pump the potentially harmful agents out faster," says Dmitry Knorre, a co-author of the work, senior research scientist at Belozersky Institute of Physico-Chemical Biology. "We hypothesized that lipophilic cations would not cause the compensatory response. Due to their positive charge, they enter negatively charged cell organelles (mitochondria) and are accumulated there. Therefore, these substances would not be presented at high concentration in the cytoplasm of a treated cell. Fungi xenobiotic sensors operate in cytoplasm according to our knowledge. We expected that the cells would be unable to detect the xenobiotics inside mitochondria, and therefore there would be no hyperactivation of ABC-transporters."
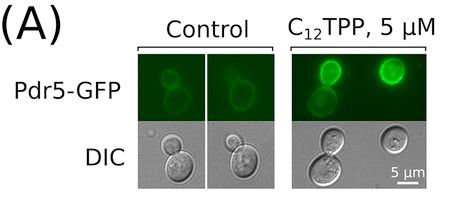
To verify this hypothesis, the researchers added a lipophilic cation called dodecyltriphenylphosphonium (C12TPP) to baker's yeast. These well known yeast species often used as model organism for studying molecular mechanisms in fungi. For the convenience of the study, the scientists generated a hybrid protein—one of its parts was a major fungal ABC-transporter, and the other one was a green fluorescent protein. When irradiated, this compound emits light so its accumulation in the cells can be observed via a fluorescence microscope. The second part did not interfere with the enzymatic activity, the protein played the role of the ABC-transporter in the cell.
The study showed that the interaction with lipophilic cations still makes the cells to initiate the compensatory response. In the presence of the lipophilic cations, the yeast produced and accumulated significantly more of the ABC-transporters than under the regular conditions. The other methods also indicated the increase of multiple drug resistance upon the treatment with the lipophilic cations. The mechanism that helps a cell to detect xenobiotics in mitochondria is still unknown. It is likely to involve several complementary systems that react to potentially harmful substances. The principles of their functioning are still to be determined. The authors plan to include pathogenic fungi in further studies.
More information: Kseniia V. Galkina et al, Penetrating cations induce pleiotropic drug resistance in yeast, Scientific Reports (2018). DOI: 10.1038/s41598-018-26435-z
Journal information: Scientific Reports
Provided by Lomonosov Moscow State University