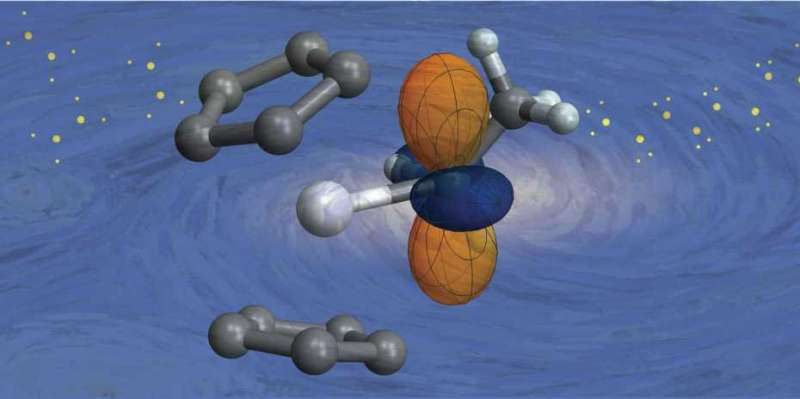
The electronic nature of a molecule determines its properties and reactivity. The image illustrates a catalyst producing polyethylene. Credit: Christopher Gordon / ETH Zürich
An international team of chemists has found a method to accelerate the development of new catalysts. Using NMR spectroscopy together with computational chemisty, they can evaluate whether or not molecules can enable reactions.
About 90 percent of all chemical processes in industry depend on catalysts – molecules that enable or accelerate chemical reactions, thus allowing them to take place at lower temperatures. The analogy from nature are enzymes that carry out complex biochemical transformations in the organism selectively and very efficiently.
In industry, catalysts are essential for saving energy, making processes more sustainable and therefore cost efficient. There is obviously a huge interest in discovering such new reaction enablers to produce chemicals and materials in a more efficient way. However, catalyst development is still very empirical nowadays, heavily relying on trial and error, and sometimes luck.
Understanding catalysts in detail
In order to develop new catalysts and make them more efficient, it is important to understand the distribution and binding ability of their electrons in detail. This electronic structure determines the character of molecules, for example the color, the smell, but also the reactivity. If the exact electronic structure of a compound is known, it is also possible to make predictions about its chemical properties.
This is exactly what researchers in Prof. Copéret's group, in collaboration with an international team, have now realized: Using nuclear magnetic resonance (NMR) spectroscopy – one of the most common analytical methods in chemistry – in combination with state-of-the-art computational chemistry, they can now gain insight into the electronic structure of catalysts and predict their reactivity. This new methodology that they just published in PNAS will make the design and discovery of catalysts easier and less dependent on screening and serendipity.
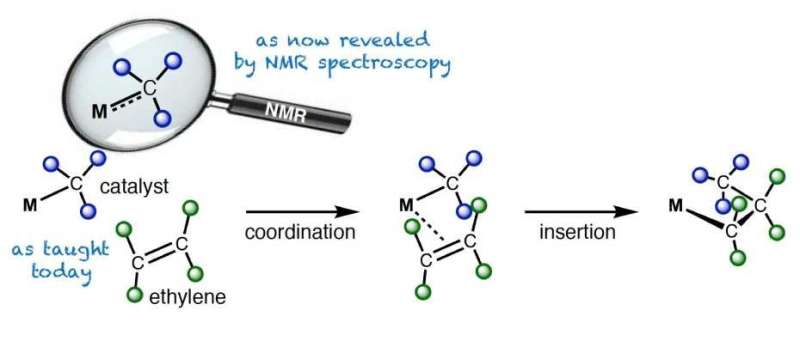
Polymerization of ethylene with organometallic catalysts (the M stands for the metal, typically Ti, Zr or Hf) as discussed in textbooks (bottom). The magnifying glass above illustrates the new findings revealed by NMR spectroscopy: the metal-carbon bond has a double bond character. Credit: Christopher Gordon / ETH Zürich
Polymerizing ethylene
In their study, the researchers investigated catalysts that are used in industry to polymerize olefins. Polyolefins are commodity chemicals such as polypropylene and polyethylene. Their applications range from packaging and fishing nets to high-end products such as bulletproof vests. Polyethylene is produced by polymerizing ethylene in the presence of so-called organometallic catalysts – molecules that contain a metal bonded to at least one carbon atom.
In basic chemistry lectures, students learn that there are single, double and triple bonds in molecules. And they learn that polyolefins are produced by catalysts that contain a metal-carbon single bond. However, this study shows that reality is not always so simple: In the investigated class of catalysts, the carbon-metal bond lies between a single bond and a double bond, depending on the metal and charge.
Double bond character determines reactivity
The degree of this double bond is decisive for the catalytic activity. It was exactly this double bond character that the researchers were now able to derive from NMR spectroscopy directly from the chemical shift of the carbon atom. They could show that the more the bond between the metal and carbon atoms behaves like a double bond, the easier a catalyst produces polyolefins. Researchers had so far not understood this fact which provides a counterintuitive conclusion: the more double bond character the metal-carbon bond has, the shorter and stronger it is – nevertheless, the easier it is to break it during olefin polymerization.
Designing catalysts faster
By combining NMR spectroscopy with theoretical calculations, it is now possible to predict whether a catalyst will enable a chemical reaction. The researchers expect that this new method will provide chemists with a finer understanding of the electronic structure of catalysts and accelerate catalyst design in the future.
More information: Christopher P. Gordon et al. NMR chemical shift analysis decodes olefin oligo- and polymerization activity of d0group 4 metal complexes, Proceedings of the National Academy of Sciences (2018). DOI: 10.1073/pnas.1803382115
Journal information: Proceedings of the National Academy of Sciences
Provided by ETH Zurich