Self-heating drinks cans set for a relaunch—here's how they work

A US technology firm is hoping to make a very old idea finally work by launching self-heating drinks cans. HeatGenie recently received US$6m to bring their can design to market in 2018, more than 15 years after Nestle abandoned a similar idea. Yet the principles behind the technology go back much further to 1897, when Russian engineer Yevgeny Fedorov invented the first self-heating can. So how do these cans work, why no one has managed to make them a success, and what's HeatGenie's new approach? To answer that, we have to go back to World War II.
The imposing cliffs of Pointe de Hoc overlook the Normandy beaches where Allied troops landed on June 6 1944. The assaults marked the beginning of the liberation of German occupied Europe. And the cliff tops were the perfect spot for artillery pieces capable of devastating any troops who tried to attack the Ohama and Utah beachheads.
The Allied command knew this and so, to sure up the attack, the navy bombarded Pointe de Hoc. Afraid this might not be enough, they also had a backup plan. A team of US Rangers scaled the shear 30-metre cliffs and, after locating the weaponry, they deployed their grenades, destroying the guns. The key to their success was the choice of thermite-based charges. These weren't the kind of "high explosives" normally found in grenades, but instead used a chemical reaction that produced temperatures hot enough to melt the steel of the artilleries' firing mechanisms.
Surprisingly, the thermite the rangers used is incredibly simple. It is just rust (iron oxide) and powdered aluminium. Mixed together they are entirely safe and stable – that is until the mixture is given an energetic kick, typically by lighting a magnesium metal fuse. And then the fireworks start. The aluminium grabs the oxygen from the rust and in the process produces iron and a huge about of heat. The reaction can easily reach 2,500℃, hot enough to produce molten (liquid) iron.
The following video shows the reaction in slow motion. The bright light at the start is just the magnesium burning. Then, when the fuse burns down to the thermite, things get impressive, leaving a melted tube and a flaming puddle of iron.
Thermite is an extreme example of an exothermic reaction, a chemical reaction that produces energy in the form of light and heat. Fire, typically the result of a reacting carbon and oxygen, is probably the exothermic reaction we are most familiar with. But there are plenty more. In fact many of the very same troops who were landing on the Normandy beaches that day had another example in their ration packs, in the form of self-heating cans of soup.
These were essentially a stove and can rolled into one, with a tube of cordite (more typically used as the propellant in small arms ammunition) running through the centre of the can to act as fuel. The cans were quick and easy to use and could be lit with a cigarette, allowing troops to prepare a hot meat in under five minutes. Unfortunately, they also had a tendency to explode, showering the assembled squaddies with piping hot soup.
Since then, there have been numerous attempts to make self-heating cans into a mainstream product. Most relied on a rather less explosive reaction to provide the heat, although some have still struggled with the problem of not blowing up. Quicklime (calcium oxide) heats up rapidly when mixed with water. But it's not particularly efficient, producing about 60 calories of energy per gram of reactant (one calorie will heat up one millilitre of water by 1℃).
The upshot is that, to heat the drink by 40℃, you need a heating element that takes up nearly half the packaging. That's just about OK if you want a small drink on a warm day, but in the depths of winter, when you might really want a hot drink, you only end up with a tepid coffee.

More powerful cans
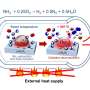
What's needed is a much more efficient reaction. Something, like thermite perhaps? As crazy as packing a can with a reaction capable of disabling an artillery gun may seem that's just what HeatGenie is planning. Over the last ten years, the firm has filed numerous patents describing the use of thermite within self-heating cans. It turns out the reaction used by the US Rangers is still too hot to handle, so they've dialled things back a bit by replacing the rust with a less reactive but no less familiar material, silicon dioxide. So the latest generation of heated cans is fuelled on aluminium and ground-up glass.
When this reaction is triggered it still kicks out a whopping 200 calories per gram of reactant and can achieve 1,600℃. Given the troubled history of self-heating packaging, releasing this much energy from the can in your hand might be a bit of a concern, so several of HeatGenie's patents cover safety issues.
These include a complex arrangement of "firewalls" that can block the so-called "flamefront" should things get too hot, and energy-absorbing "heatsinks" to ensure the heat is efficiently transmitted around the drink, as well as vents to let off any steam. With all that is place, the company claims just 10% of the packaging is taken up by the heating elements, which can still produce a warm coffee in two minutes (although the exact temperature hasn't been revealed).
So, well over a century on from Fedorov's first efforts, has HeatGenie final cracked the self-heating can? Judging from the patents and investments, the firm might have sorted out the technical side, but whether it really has a hot product on its hands is another thing entirely.
Provided by The Conversation
This article was originally published on The Conversation. Read the original article.![]()